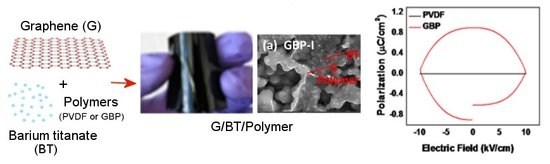
Advancing Dielectric and Ferroelectric Properties of Piezoelectric Polymers by Combining Graphene and Ferroelectric Ceramic Additives for Energy Storage Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Graphene/Barium Titanate/Polyvinylidene Fluoride (G/BT/PVDF) Nanocomposite Films
2.3. Characterizations
3. Results and Discussion
3.1. Characterization of Graphene/Barium Titanate/Polyvinylidene Fluoride (G/BT/PVDF) Nanocomposite Films
3.2. Dielectric Studies of G/BT/PVDF Nanocomposite Films
3.3. Ferroelectric Studies of G/BT/PVDF Nanocomposite Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ribeiro, C.; Sencadas, V.; Correia, D.M.; Lanceros-Méndez, S. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf. B Biointerfaces 2015, 136, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Zhao, P.; McConohy, G.; Yang, H.; Tong, Y.; Wang, X. Sponge-like piezoelectric polymer films for scalable and integratable nanogenerators and self-powered electronic systems. Adv. Energy Mater. 2014, 4, 1301624. [Google Scholar] [CrossRef]
- Vanden Ende, D.A.; VandeWiel, H.J.; Groen, W.A.; Vander Zwaag, S. Direct strain energy harvesting in automobile tires using piezoelectric PZT–polymer composites. Smart Mater. Struct. 2012, 21, 015011. [Google Scholar] [CrossRef]
- Ortiz, R.P.; Facchetti, A.; Marks, T.J. High-k organic, inorganic, and hybrid dielectrics for low-voltage organic field-effect transistors. Chem. Rev. 2010, 110, 205–239. [Google Scholar] [CrossRef] [PubMed]
- Hao, X. A review on the dielectric materials for high energy-storage application. J. Adv. Dielectr. 2013, 3, 1330001. [Google Scholar] [CrossRef]
- Carpi, F.; Salaris, C.; Derossi, D. Folded dielectric elastomer actuators. Smart Mater. Struct. 2007, 16, S300. [Google Scholar] [CrossRef]
- Ramadan, K.S.; Sameoto, D.; Evoy, S. A review of piezoelectric polymers as functional materials for electromechanical transducers. Smart Mater. Struct. 2014, 23, 33001–33026. [Google Scholar] [CrossRef]
- Matko, V.; Milanović, M. Temperature-compensated capacitance–frequency converter with high resolution. Sens. Actuators A 2014, 220, 262–269. [Google Scholar] [CrossRef]
- Matko, V. Next generation AT-cut quartz crystal sensing devices. Sensors 2011, 5, 4474–4482. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.M.; Zhou, T.; Yao, S.H.; Yuan, J.K.; Zha, J.W.; Song, H.T.; Li, J.Y.; Chen, Q.; Yang, W.T.; Bai, J. Advanced calcium copper titanate/polyimide functional hybrid films with high dielectric permittivity. Adv. Mater. 2009, 21, 2077–2082. [Google Scholar] [CrossRef]
- Tang, H.; Lin, Y.; Andrews, C.; Sodano, H.A. Nanocomposites with increased energy density through high aspect ratio PZT nanowires. Nanotechnology 2011, 22, 015702. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, H.I.; Lin, K.Y.; Yen, F.S.; Hwang, C.Y. Effects of particle size of BaTiO3 powder on the dielectric properties of BaTiO3/polyvinylidene fluoride composites. J. Mater. Sci. 2001, 36, 3809–3815. [Google Scholar] [CrossRef]
- Sugumaran, S.; Bellan, C.S. Transparent nano composite PVA–TiO2 and PMMA–TiO2 thin films: Optical and dielectric properties. Optik 2014, 125, 5128–5133. [Google Scholar] [CrossRef]
- Rao, Y.; Ogitani, S.; Kohl, P.; Wong, C. Novel polymer–ceramic nanocomposite based on high dielectric constant epoxy formula for embedded capacitor application. J. Appl. Polym. Sci. 2002, 83, 1084–1090. [Google Scholar] [CrossRef]
- Upadhyay, R.H.; Deshmukh, R.R. Investigation of dielectric properties of newly prepared β-phase polyvinylidene fluoride–barium titanate nanocomposite films. J.Electrostat. 2013, 71, 945–950. [Google Scholar] [CrossRef]
- Arbatti, M.; Shan, X.; Cheng, Z.Y. Ceramic–polymer composites with high dielectric constant. Adv. Mater. 2007, 19, 1369–1372. [Google Scholar] [CrossRef]
- Yao, S.H.; Dang, Z.M.; Jiang, M.J.; Bai, J. BaTiO3-carbon nanotube/polyvinylidene fluoride three-phase composites with high dielectric constant and low dielectric loss. Appl. Phys. Lett. 2008, 93, 3502. [Google Scholar] [CrossRef]
- Zhang, C.; Chi, Q.; Dong, J.; Cui, Y.; Wang, X.; Liu, L.; Lei, Q. Enhanced dielectric properties of poly (vinylidene fluoride) composites filled with nano iron oxide-deposited barium titanate hybrid particles. Sci. Rep. 2016, 6, 33508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, D.; Ma, J. Dielectric properties of PVDF/Ag/BaTiO3composites. Ferroelectrics 2013, 455, 77–82. [Google Scholar] [CrossRef]
- Tong, W.; Zhang, Y.; Yu, L.; Luan, X.; An, Q.; Zhang, Q.; Lv, F.; Chu, P.K.; Shen, B.; Zhang, Z. Novel method for the fabrication of flexible film with oriented arrays of graphene in poly (vinylidene fluoride-co-hexafluoropropylene) with low dielectric loss. J. Appl. Gerontol. 2014, 29, 434–454. [Google Scholar] [CrossRef]
- Li, Y.; Tjong, S.C.; Li, R. Dielectric properties of binary polyvinylidene fluoride/barium titanate nanocomposites and their nanographite doped hybrids. Express Polym. Lett. 2011, 5, 526–534. [Google Scholar] [CrossRef]
- Dang, Z.M.; Wang, L.; Yin, Y.; Zhang, Q.; Lei, Q.Q. Giant dielectric permittivities in functionalized carbon-nanotube/electroactive-polymer nanocomposites. Adv. Mater. 2007, 19, 852–857. [Google Scholar] [CrossRef]
- He, F.; Lau, S.; Chan, H.L.; Fan, J. High dielectric permittivity and low percolation threshold in nanocomposites based on poly (vinylidene fluoride) and exfoliated graphite nanoplates. Adv. Mater. 2010, 21, 710–715. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, T.; Zha, J.W.; Zhao, J.; Shi, C.Y.; Dang, Z.M. Functionalized graphene–BaTiO3/ferroelectric polymer nanodielectric composites with high permittivity, low dielectric loss, and low percolation threshold. J. Mater. Chem. A 2013, 1, 6162–6168. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, U.; Chung, G.S. Synthesis and characterization of the PVDF-BTO nanocomposites with the employment of RGO sheets for flexible energy harvesters. Procedia Eng. 2016, 168, 1074–1077. [Google Scholar] [CrossRef]
- Li, W.; Meng, Q.; Zheng, Y.; Zhang, Z.; Xia, W.; Xu, Z. Electric energy storage properties of poly (vinylidene fluoride). Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Vinogradov, A.; Holloway, F. Electro-mechanical properties of the piezoelectric polymer PVDF. Ferroelectrics 1999, 226, 169–181. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite-and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Yaqoob, U.; Uddin, A.I.; Chung, G.S. The effect of reduced graphene oxide on the dielectric and ferroelectric properties of PVDF–BaTiO3 nanocomposites. RSC Adv. 2016, 6, 30747–30754. [Google Scholar] [CrossRef]
- Radwan, R.M.; Aly, S.S.; Abd El Aal, S. Preparation, characterization and effect of electron beam irradiation on the structure and dielectric properties of BatiO3/PVDF composite films. J.Radiat. Res. Appl. Sci. 2008, 11, 9–16. [Google Scholar]
- Jiansirisomboon, S.; Watcharapasorn, A.; Tunkasiri, T. Fabrication, microstructure and mechanical properties relations of ferroelectric barium titanate reinforced with alumina micro/nano particulates. Adv. Sci. Technol. 2006, 45, 2406–2411. [Google Scholar] [CrossRef]
- Joseph, N.; Singh, S.K.; Sirugudu, R.K.; Murthy, V.R.K.; Ananthakumar, S.; Sebastian, M.T. Effect of silver incorporation into PVDF-barium titanate composites for EMI shielding applications. Mater. Res. Bull. 2013, 48, 1681–1687. [Google Scholar] [CrossRef]
- Frey, M.; Payne, D. Grain-size effect on structure and phase transformations for barium titanate. Phys. Rev. BCondens. Matter 1996, 54, 3158. [Google Scholar] [CrossRef]
- Pant, H.C.; Patra, M.K.; Verma, A.; Vadera, S.R.; Kumar, N. Study of the dielectric properties of barium titanate–polymer composites. Acta Mater. 2006, 54, 3163–3169. [Google Scholar] [CrossRef]
- Yu, L.; Cebe, P. Crystal polymorphism in electrospun composite nanofibers of poly (vinylidene fluoride) with nanoclay. Polymer 2009, 50, 2133–2141. [Google Scholar] [CrossRef]
- Loryuenyong, V.; Totepvimarn, K.; Eimburanapravat, P.; Boonchompoo, W.; Buasri, A. Preparation and characterization of reduced graphene oxide sheets via water-based exfoliation and reduction methods. Adv. Mater. Sci. Eng. 2013, 2013, 923403. [Google Scholar] [CrossRef]
- Chrissafis, K.; Bikiaris, D. Can nanoparticles really enhance thermal stability of polymers? Part I: An overview on thermal decomposition of addition polymers. Thermochim. Acta 2011, 523, 1–24. [Google Scholar] [CrossRef]
- Dang, Z.M.; Yuan, J.K.; Zha, J.W.; Zhou, T.; Li, S.T.; Hu, G.H. Fundamentals, processes and applications of high-permittivity polymer–matrix composites. Prog. Mater Sci. 2012, 57, 660–723. [Google Scholar] [CrossRef]
- Shah, S.M.H.; Akbar, A.; Riaz, S.; Atiq, S.; Naseem, S. Magnetic, structural, and dielectric properties of Bi1-xKxFeO3 thin films using sol-gel. IEEE Trans.Magn. 2014, 50, 1–4. [Google Scholar]
- Barzic, R.F.; Barzic, A.I.; Dumitrascu, G. Percolation effects on dielectric properties of polystyrene/BaTiO3 nanocomposites. UPB Sci. Bull. 2014, 76, 225–234. [Google Scholar]
- Hao, Y.; Wang, X.; O’Brien, S.; Lombardi, J.; Li, L. Flexible BaTiO3/PVDF gradated multilayer nanocomposite film with enhanced dielectric strength and high energy density. J. Mater. Chem. C 2015, 3, 9740–9747. [Google Scholar] [CrossRef]
- Song, Y.; Shen, Y.; Liu, H.; Lin, Y.; Li, M.; Nan, C.W. Enhanced dielectric and ferroelectric properties induced by dopamine-modified BaTiO3 nanofibers in flexible poly (vinylidene fluoride-trifluoroethylene) nanocomposites. J. Mater. Chem. 2012, 22, 8063–8068. [Google Scholar] [CrossRef]
- Cho, S.; Kim, M.; Lee, J.S.; Jang, J. Polypropylene/polyaniline nanofiber/reduced graphene oxide nanocomposite with enhanced electrical, dielectric, and ferroelectric properties for a high energy density capacitor. ACS Appl.Mater. Interfaces 2015, 7, 22301–22314. [Google Scholar] [CrossRef] [PubMed]
- Nan, C.W.; Shen, Y.; Ma, J. Physical properties of composites near percolation. Annu. Rev. Mater. Sci. 2010, 40, 131–151. [Google Scholar] [CrossRef]
- Lakshmi, N.; Tambe, P.; Sahu, N.K. Giant permittivity of three phase polymer nanocomposites obtained by modifying hybrid nanofillers with polyvinylpyrrolidone. Compos. Interfaces 2017, 455, 1–21. [Google Scholar] [CrossRef]
- Qi, F.; Chen, N.; Wang, Q. Dielectric and piezoelectric properties in selective laser sintered polyamide11/BaTiO3/CNT ternary nanocomposites. Mater. Des. 2018, 143, 72–80. [Google Scholar] [CrossRef]
- Li, M.; Huang, X.; Wu, C.; Xu, H.; Jiang, P.; Tanaka, T. Fabrication of two-dimensional hybrid sheets by decorating insulating PANI on reduced graphene oxide for polymer nanocomposites with low dielectric loss and high dielectric constant. J. Mater. Chem. 2012, 22, 23477–23484. [Google Scholar] [CrossRef]
- Pan, Z.; Yao, L.; Zhai, J.; Shen, B.; Wang, H. Significantly improved dielectric properties and energy density of polymer nanocomposites via small loaded of BaTiO3 nanotubes. Compos. Sci. Technol. 2017, 147, 30–38. [Google Scholar] [CrossRef]
- Barrau, S.; Demont, P.; Peigney, A.; Laurent, C.; Lacabanne, C. DC and AC conductivity of carbon nanotubes−polyepoxy composites. Macromolecules 2003, 36, 5187–5194. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, D.; Samantaray, B.; Choudhary, R.; Thakur, A. Complex impedance analysis of layered perovskite structure electroceramics—NaDyTiO4. J. Mater. Sci. 2005, 40, 5419–5425. [Google Scholar] [CrossRef]
- Singh, G.; Tiwari, V. Effect of Zr concentration on conductivity behavior of (1− x) PMN-xPZ ceramic: An impedance spectroscopy analysis. J. Appl. Phys. 2009, 106, 1380. [Google Scholar] [CrossRef]
- Ataur Rahman, M.; Chung, G.S. Synthesis of PVDF-graphene nanocomposites and their properties. J. Alloys Compd. 2013, 581, 724–730. [Google Scholar] [CrossRef]
- Ounaies, Z.; Park, C.; Wise, K.; Siochi, E.; Harrison, J. Electrical properties of single wall carbon nanotube reinforced polyimide composites. Compos. Sci. Technol. 2003, 63, 1637–1646. [Google Scholar] [CrossRef]









| Sample Code | Weight of Graphene(mg) | Weight of BT (mg) | Weight of PVDF (mg) | Weight Ratio of G:BT:PVDF |
|---|---|---|---|---|
| GBP-I | 50 | 100 | 1000 | 0.05:0.1:1 |
| GBP-II | 100 | 100 | 1000 | 0.1:0.1:1 |
| GBP-III | 150 | 100 | 1000 | 0.15:0.1:1 |
| GBP-IV | 200 | 100 | 1000 | 0.2:0.1:1 |
| GBP-V | 150 | 200 | 1000 | 0.15:0.2:1 |
| GBP-VI | 150 | 300 | 1000 | 0.15:0.3:1 |
| GBP-VII | 150 | 400 | 1000 | 0.15:0.4:1 |
| GBP-VIII | 150 | 500 | 1000 | 0.15:0.5:1 |
| GBP-IX | 150 | 600 | 1000 | 0.15:0.6:1 |
| GBP-X | 150 | 700 | 1000 | 0.15:0.7:1 |
| Dielectric Material | Frequency (Hz) | Dielectric Constant (ɛ’) | Loss Tangent (tanδ) | References |
|---|---|---|---|---|
| PBCNCs-3D | 1000/100 | 16.2 | 0.15 | [49] |
| PMMA/rPANI@rGO | 1000 | 40 | 0.12 | [50] |
| PVA/TiO2 | 1000 | 24.6 | 0.1–1 | [12] |
| PMMA/TiO2 | 1000 | 26.8 | 0.1–0.8 | [12] |
| PMN-PT/BaTiO3/Epoxy | 10000 | 110 | 0.016 | [13] |
| PVDF/Graphite | 1000 | 4.5 × 107 | 229 | [23] |
| BTNTs/PVDF | 100 | 47.05 | 0.1 | [51] |
| G/BT/PVDF | 40 | 199 | 0.6 | This work |
| G/BT/PVDF | 106 | 22.5 | 0.05 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishaq, S.; Kanwal, F.; Atiq, S.; Moussa, M.; Azhar, U.; Imran, M.; Losic, D. Advancing Dielectric and Ferroelectric Properties of Piezoelectric Polymers by Combining Graphene and Ferroelectric Ceramic Additives for Energy Storage Applications. Materials 2018, 11, 1553. https://doi.org/10.3390/ma11091553
Ishaq S, Kanwal F, Atiq S, Moussa M, Azhar U, Imran M, Losic D. Advancing Dielectric and Ferroelectric Properties of Piezoelectric Polymers by Combining Graphene and Ferroelectric Ceramic Additives for Energy Storage Applications. Materials. 2018; 11(9):1553. https://doi.org/10.3390/ma11091553
Chicago/Turabian StyleIshaq, Saira, Farah Kanwal, Shahid Atiq, Mahmoud Moussa, Umar Azhar, Muhammad Imran, and Dusan Losic. 2018. "Advancing Dielectric and Ferroelectric Properties of Piezoelectric Polymers by Combining Graphene and Ferroelectric Ceramic Additives for Energy Storage Applications" Materials 11, no. 9: 1553. https://doi.org/10.3390/ma11091553