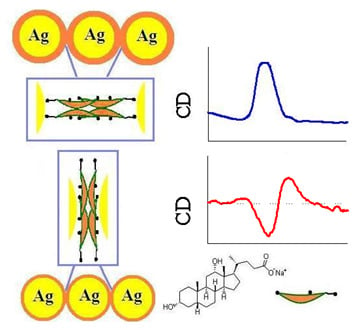
Synthesis and Plasmonic Chiroptical Studies of Sodium Deoxycholate Modified Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Situ Preparation of NaDC-Capped Ag NPs
2.3. Preparation of NaDC-Capped Ag NPs by Ligand Exchange Reaction
2.4. Preparation of Ag NPs in NaDC and HDC Mixed Solutions
2.5. Ex Situ Preparation of NaDC-Capped Ag NPs under the Acidic Condition
2.6. Characterization
3. Results and Discussions
3.1. Chiroptical Property of In Situ Prepared NaDC-Capped Ag NPs at Neutral pH
3.2. Chiroptical Property of Ex Situ Prepared NaDC-Capped Ag NPs
3.3. Chiroptical Properties of Prepared NaDC-Capped Ag NPs under the Acidic Conditions
3.4. Mechanism of the Interactions between Chiral Molecules and Ag NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bjerneld, E.J.; Svedberg, F.; Käll, M. Laser-induced growth and deposition of noble-metal nanoparticles for Surface-Enhanced Raman Scattering. Nano Lett. 2003, 3, 593–596. [Google Scholar] [CrossRef]
- Wang, X.J.; Wang, C.; Cheng, L.; Lee, S.T.; Liu, Z. Noble metal coated single-walled carbon nanotubes for applications in surface enhanced raman scattering imaging and photothermal therapy. J. Am. Chem. Soc. 2012, 134, 7414–7422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z. Biomedical applications of shape-controlled plasmonic nanostructures: A case study of hollow gold nanospheres for photothermal ablation therapy of cancer. J. Phys. Chem. Lett. 2010, 1, 686–695. [Google Scholar] [CrossRef]
- Wu, Q.Z.; Cao, H.Q.; Luan, Q.Y.; Zhang, J.Y.; Wang, Z.; Warner, J.H.; Watt Andrew, A.R. Biomolecule-assisted synthesis of water-soluble silver nanoparticles and their biomedical applications. Inorg. Chem. 2008, 47, 5882–5888. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Fujihara, H. Chiral bisphosphine BINAP-stabilized gold and palladium nanoparticles with small size and their palladium nanoparticle-catalyzed asymmetric reaction. J. Am. Chem. Soc. 2003, 125, 15742–15743. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.F.; Wang, Z.G.; Li, N.; Ding, B.Q. Engineering gold nanoparticles with DNA ligands for selective catalytic oxidation of chiral substrates. ACS Catal. 2015, 5, 1489–1498. [Google Scholar] [CrossRef]
- Wei, J.J.; Guo, Y.J.; Li, J.Z.; Yuan, M.K.; Long, T.F.; Liu, Z.D. Optically active ultrafine Au–Ag alloy nanoparticles used for colorimetric chiral recognition and circular dichroism sensing of enantiomers. Anal. Chem. 2017, 89, 9781–9787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, C.L.; Song, G.X.; Li, B.X. Self-assembly of L-cysteine–gold nanoparticles as chiral probes for visual recognition of 3,4-dihydroxyphenylalanine enantiomers. RSC Adv. 2015, 5, 27003–27008. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, L.G.; Liz-Marzán, L.M.; Ma, W.; Kotov, N.A.; Wang, L.B.; Kuang, H.; Xu, C.L. Sensitive detection of silver ions based on chiroplasmonic assemblies of nanoparticles. Adv. Opt. Mater. 2013, 1, 626–630. [Google Scholar] [CrossRef]
- Abdulrahman, N.A.; Fan, Z.; Tonooka, T.; Kelly, S.M.; Gadegaard, N.; Hendry, E.; Govorov, A.O.; Kadodwala, M. Induced chirality through electromagnetic coupling between chiral molecular layers and plasmonic nanostructures. Nano Lett. 2012, 12, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.J.; Wang, Y.L.; Zhuang, H.; Zhang, J.H. DNA-engineered chiroplasmonic heteropyramids for ultrasensitive detection of mercury ion. Biosens. Bioelectron. 2015, 68, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, A.; Schreiber, R.; Fan, Z.Y.; Pardatscher, G.; Roller, E.; Högele, A.; Simmel, F.C.; Govorov, A.O.; Liedl, T. DNA-based self-assembly of chiral plasmonic nanostructures with tailored optical response. Nature 2012, 483, 311–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.J.; Xu, L.G.; Xu, C.L.; Ma, W.; Kuang, H.; Wang, L.B.; Kotov, N.A. Self-assembly of chiral nanoparticle pyramids with strong R/S optical activity. J. Am. Chem. Soc. 2012, 134, 15114–15121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lan, X.; Chiu, Y.C.; Lu, X.X.; Ni, W.H.; Gao, H.W.; Wang, Q.B. Strong chiroptical activities in gold nanorod dimers assembled using DNA origami templates. ACS Photonics 2015, 2, 392–397. [Google Scholar] [CrossRef]
- Shen, X.B.; Song, C.; Wang, J.Y.; Shi, D.W.; Wang, Z.G.; Liu, N.; Ding, B.Q. Rolling up gold nanoparticle-dressed DNA origami into three-dimensional plasmonic chiral nanostructures. J. Am. Chem. Soc. 2012, 134, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Wang, X.S.; Yin, H.; Shi, Y.; Jiang, H.J.; Chen, T.R.; Gao, J.X.; Qu, D.; Xu, Y.; Ding, D.J. Free-standing optically switchable chiral plasmonic photonic crystal based on self-assembled cellulose nanorods and gold nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 21797–21806. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Yang, S.; Bao, W.; Kao, J.; Thorkelsson, K.; Salmeron, M.; Zhang, X.; Xu, T. Diversifying nanoparticle assemblies in supramolecule nanocomposites via cylindrical confinement. Nano Lett. 2017, 17, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Lukach, A.; Thérien-Aubin, H.; Querejeta-Fernández, A.; Pitch, N.; Chauve, G.; Méthot, M.; Bouchard, J.; Kumacheva, E. Coassembly of gold nanoparticles and cellulose nanocrystals in composite films. Langmuir 2015, 31, 5033–5041. [Google Scholar] [CrossRef] [PubMed]
- Rezanka, P.; Zaruba, K.; Kral, V. Supramolecular chirality of cysteine modified silver nanoparticles. Coll. Surf. A Physicochem. Eng. Asp. 2011, 374, 77–83. [Google Scholar] [CrossRef]
- Wang, R.Y.; Wang, P.; Liu, Y.N.; Zhao, W.J.; Zhai, D.W.; Hong, X.H.; Ji, Y.L.; Wu, X.C.; Wang, F. Experimental observation of giant chiroptical amplification of small chiral molecules by gold nanosphere clusters. J. Phys. Chem. C 2014, 118, 9690–9695. [Google Scholar]
- Leroux, F.; Gysemans, M.; Bals, S.; Batenburg, K.J.; Snauwaert, J.; Verbiest, T.; VanHaesendonck, C.; Tendeloo, G.V. Three-dimensional characterization of helical silver nanochains mediated by protein assemblies. Adv. Mater. 2010, 22, 2193–2197. [Google Scholar] [CrossRef] [PubMed]
- Shemer, G.; Krichevski, O.; Markovich, G.; Molotsky, T.; Lubitz, I.; Kotlyar, A.B. Chirality of silver nanoparticles synthesized on DNA. J. Am. Chem. Soc. 2006, 128, 11006–11007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Govorov, A.O. Giant Circular dichroism of a molecule in a region of strong plasmon resonances between two neighboring gold nanocrystals. Phys. Rev. B 2012, 138–143. [Google Scholar] [CrossRef]
- Ge′rard, V.A.; Gun’ko, Y.K.; Defrancq, E.; Govorov, A.O. Plasmon-induced CD response of oligonucleotide-conjugated metal Nanoparticles. Chem. Commun. 2011, 47, 7383–7385. [Google Scholar] [CrossRef] [PubMed]
- Roman-Velazquez, C.E.; Noguez, C.; Garzon, I.L. Circular dichroism simulated spectra of chiral gold nanoclusters: A dipole approximation. J. Phys. Chem. B 2003, 107, 12035–12308. [Google Scholar] [CrossRef]
- Yao, H.; Miki, K.; Nishida, N.; Sasaki, A.; Kimura, K. Large optical activity of gold nanocluster enantiomers induced by a pair of optically active penicillamines. J. Am. Chem. Soc. 2005, 127, 15536–15543. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, M.R.; George, C.B.; Zuber, G.; Naaman, R.; Waldeck, D.H.; Wipf, P.; Beratan, D.N. The chiroptical signature of achiral metal clusters induced by dissymmetric adsorbates. Phys. Chem. Chem. Phys. 2006, 8, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Gautier, C.; Bürgi, T. Chiral N-isobutyryl-cysteine protected gold nanoparticles: Preparation, size selection, and optical activity in the UV−vis and Infrared. J. Am. Chem. Soc. 2006, 128, 11079–11087. [Google Scholar] [CrossRef] [PubMed]
- Gautier, C.; Bürgi, T. L-Glutathione chemisorption on gold and acid/base induced structural changes: A PM-IRRAS and time-resolved in situ ATR-IR spectroscopic study. Langmuir 2005, 21, 1354–1363. [Google Scholar]
- Govorov, A.O.; Fan, Z.Y.; Hernandez, P.; Slocik, J.M.; Naik, R.R. Theory of circular dichroism of nanomaterials comprising chiral molecules and nanocrystals: Plasmon enhancement, dipole interactions, and dielectric effects. Nano Lett. 2010, 10, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Govorov, A.O. Plasmon-induced circular dichroism of a chiral molecule in the vicinity of metal nanocrystals. Application to various geometries. J. Phys. Chem. C 2011, 115, 7914–7923. [Google Scholar] [CrossRef]
- Nair, P.P.; Kritchevsky, D.; Setchell, K.D.R. (Eds.) The Bile Acid: Chemistry, Physiology and Metabolism; Plenum Press: New York, NY, USA, 1971; pp. 176–177. ISBN 978-1-4757-0649-9. [Google Scholar]
- Layani, M.E.; Moshe, A.B.; Varenik, M.; Regev, O.; Zhang, H.; Govorov, A.O.; Markovich, G. Chiroptical activity in silver cholate nanostructures induced by the formation of nanoparticle assemblies. J. Phys. Chem. C 2013, 117, 22240–22244. [Google Scholar] [CrossRef]
- Zhu, Z.N.; Liu, W.J.; Li, Z.T.; Han, B.; Zhou, Y.L.; Gao, Y.; Tang, Z.Y. Manipulation of collective optical activity in one-dimensional plasmonic assembly. ACS Nano 2012, 6, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Martíneza, A.; Alonso-Gómezb, J.L.; Auguiéa, B.; Cidb, M.M.; Liz-Marzána, L.M. From individual to collective chirality in metal nanoparticles. Nano Today 2011, 6, 381–400. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Fei, K.-X.; Yang, X.; Zhang, S.-S.; Peng, Y.-X. Synthesis and Plasmonic Chiroptical Studies of Sodium Deoxycholate Modified Silver Nanoparticles. Materials 2018, 11, 1291. https://doi.org/10.3390/ma11081291
Wang J, Fei K-X, Yang X, Zhang S-S, Peng Y-X. Synthesis and Plasmonic Chiroptical Studies of Sodium Deoxycholate Modified Silver Nanoparticles. Materials. 2018; 11(8):1291. https://doi.org/10.3390/ma11081291
Chicago/Turabian StyleWang, Jing, Kai-Xuan Fei, Xin Yang, Shuai-Shuai Zhang, and Yin-Xian Peng. 2018. "Synthesis and Plasmonic Chiroptical Studies of Sodium Deoxycholate Modified Silver Nanoparticles" Materials 11, no. 8: 1291. https://doi.org/10.3390/ma11081291