Selective Adsorption of Pb(II) from Aqueous Solution by Triethylenetetramine-Grafted Polyacrylamide/Vermiculite
Abstract
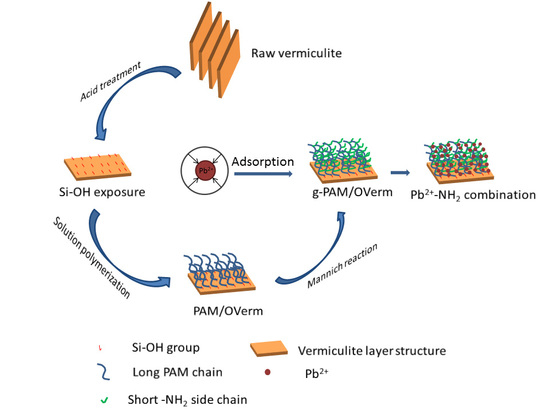
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Modification of Verm
2.3. Preparation of PAM/OVerm
2.4. Preparation of g-PAM/OVerm
2.5. Characterization
2.6. Adsorption Experiments
2.7. Regeneration of the Adsorbent
3. Results and Discussion
3.1. The Effects of the Grafting Reaction Conditions on Adsorption
3.1.1. pH of Solution
3.1.2. Temperature of the Grafting Reaction
3.1.3. Duration of the Grafting Reaction
3.2. Characterization
3.2.1. FTIR
3.2.2. SEM and EDS Analyses
3.2.3. Thermal Analysis
3.2.4. Nitrogen Adsorption–Desorption Isotherms
3.3. Adsorption of Pb(II)
3.3.1. The Effect of pH on Pb(II) Uptake
3.3.2. Study on the Adsorption Selectivity by g-PAM/OVerm
3.3.3. g-PAM/OVerm Regeneration
3.3.4. Adsorption Dynamics
3.3.5. Adsorption Isotherms
3.4. Pb(II) Adsorption Mechanism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fu, F.L.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.Q.; Liu, Y.Q.; Liu, S.B.; Zeng, G.M.; Tan, X.F.; Huang, B.Y.; Tang, X.J.; Wang, S.F.; Hua, Q.; Yan, Z.L. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Haider, S.; Park, S.Y. Preparation of the electrospun chitosan nanofibers and their applications to the adsorption of Cu(II) and Pb(II) ions from an aqueous solution. J. Membr. Sci. 2009, 328, 90–96. [Google Scholar] [CrossRef]
- Ju, X.J.; Zhang, S.B.; Zhou, M.Y.; Xie, R.; Yang, L.; Chu, L.Y. Novel heavy-metal adsorption material: Ion-recognition P(NIPAM-co-BCAm) hydrogels for removal of lead(II) ions. J. Hazard. Mater. 2009, 167, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Saikia, A.; Purkait, M.K.; Mohanty, K. Chitosan based ceramic ultrafiltration membrane: Preparation, characterization and application to remove Hg(II) and As(III) using polymer enhanced ultrafiltration. Chem. Eng. J. 2011, 170, 209–219. [Google Scholar] [CrossRef]
- Samiey, B.; Cheng, C.H.; Wu, J.N. Organic-Inorganic Hybrid Polymers as Adsorbents for Removal of Heavy Metal Ions from Solutions: A Review. Materials 2014, 7, 673–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Zhang, Z.H.; Liu, Y.N.; Yang, X.; Luo, L.J.; Chen, J.T.; Yao, S.Z. Preparation of core-shell magnetic ion-imprinted polymer for selective extraction of Pb(II) from environmental samples. Chem. Eng. J. 2011, 178, 443–450. [Google Scholar] [CrossRef]
- Berber-Mendoza, M.S.; Leyva-Ramos, R.; Alonso-Davila, P.; Fuentes-Rubio, L.; Guerrero-Coronado, R.M. Comparison of isotherms for the ion exchange of Pb(II) from aqueous solution onto homoionic clinoptilolite. J. Colloid Interface Sci. 2006, 301, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Veli, S.; Alyuz, B. Adsorption of copper and zinc from aqueous solutions by using natural clay. J. Hazard. Mater. 2007, 149, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Goel, J.; Kadirvelu, K.; Rajagopal, C.; Garg, V.K. Removal of lead(II) by adsorption using treated granular activated carbon: Batch and column studies. J. Hazard. Mater. 2005, 125, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Meena, A.K.; Mishra, G.K.; Rai, P.K.; Rajagopal, C.; Nagar, P.N. Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J. Hazard. Mater. 2005, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Aguado, J.; Arsuaga, J.M.; Arencibia, A.; Lindo, M.; Gascon, V. Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J. Hazard. Mater. 2009, 163, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.H.; Liu, S.C.; Chen, C.Y.; Chen, C.Y. Comparative adsorption of Cu(II), Zn(II), and Pb(II) ions in aqueous solution on the crosslinked chitosan with epichlorohydrin. J. Hazard. Mater. 2008, 154, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Sen Gupta, S.; Bhattacharyya, K.G. Adsorption of heavy metals on kaolinite and montmorillonite: A review. Phys. Chem. Chem. Phys. 2012, 14, 6698–6723. [Google Scholar] [CrossRef] [PubMed]
- Sdiri, A.; Higashi, T.; Chaabouni, R.; Jamoussi, F. Competitive Removal of Heavy Metals from Aqueous Solutions by Montmorillonitic and Calcareous Clays. Water Air Soil Pollut. 2012, 223, 1191–1204. [Google Scholar] [CrossRef]
- Tjong, S.C.; Meng, Y.Z.; Hay, A.S. Novel preparation and properties of polypropylene-vermiculite nanocomposites. Chem. Mater. 2002, 14, 44–51. [Google Scholar] [CrossRef]
- Skipper, N.T.; Soper, A.K.; McConnell, J.D.C. The structure of interlayer water in vermiculite. J. Chem. Phys. 1991, 94, 5751–5760. [Google Scholar] [CrossRef]
- Temuujin, J.; Okada, K.; MacKenzie, K.J.D. Preparation of porous silica from vermiculite by selective leaching. Appl. Clay Sci. 2003, 22, 187–195. [Google Scholar] [CrossRef]
- Hashem, F.S.; Amin, M.S.; El-Gamal, S.M.A. Chemical activation of vermiculite to produce highly efficient material for Pb2+ and Cd2+ removal. Appl. Clay Sci. 2015, 115, 189–200. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Sorgona, A.; Rizzo, M.; Cacco, G. Cadmium adsorption on vermiculite, zeolite and pumice: Batch experimental studies. J. Environ. Manag. 2009, 90, 364–374. [Google Scholar] [CrossRef]
- Stylianou, M.A.; Inglezakis, V.J.; Moustakas, K.G.; Malamis, S.P.; Loizidou, M.D. Removal of Cu(II) in fixed bed and batch reactors using natural zeolite and exfoliated vermiculite as adsorbents. Desalination 2007, 215, 133–142. [Google Scholar] [CrossRef]
- Malandrino, M.; Abollino, O.; Giacomino, A.; Aceto, M.; Mentasti, E. Adsorption of heavy metals on vermiculite: Influence of pH and organic ligands. J. Colloid Interface Sci. 2006, 299, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Jozefaciuk, G.; Bowanko, G. Effect of acid and alkali treatments on surface areas and adsorption energies of selected minerals. Clays Clay Miner. 2002, 50, 771–783. [Google Scholar] [CrossRef]
- Ravichandran, J.; Sivasankar, B. Properties and catalytic activity of acid-modified montmorillonite and vermiculite. Clays Clay Miner. 1997, 45, 854–858. [Google Scholar] [CrossRef]
- Yu, X.B.; Wei, C.H.; Ke, L.; Wu, H.Z.; Chai, X.S.; Hu, Y. Preparation of trimethylchlorosilane-modified acid vermiculites for removing diethyl phthalate from water. J. Colloid Interface Sci. 2012, 369, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.H.; Shao, D.D.; Hu, J.; Wu, W.S.; Wang, X.K. Comparison of Ni2+ sorption to bare and ACT-graft attapulgites: Effect of pH, temperature and foreign ions. Surf. Sci. 2008, 602, 778–785. [Google Scholar] [CrossRef]
- Liu, P.; Wang, T.M. Adsorption properties of hyperbranched aliphatic polyester. grafted attapulgite towards heavy metal ions. J. Hazard. Mater. 2007, 149, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Liu, H.W.; Zha, F.; Chen, H.K.; Ren, X.N.; Lei, Z.Q. Adsorption of Pb(II) by N-methylimidazole modified palygorskite. Chem. Eng. J. 2011, 167, 183–189. [Google Scholar] [CrossRef]
- Huang, J.H.; Liu, Y.F.; Wang, X.G. Selective adsorption of tannin from flavonoids by organically modified attapulgite clay. J. Hazard. Mater. 2008, 160, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Zhou, S.; Chu, X.; Yang, L.; Xing, W. Preparation and characterization of polyacrylamide/palygorskite. Appl. Clay Sci. 2009, 46, 148–152. [Google Scholar] [CrossRef]
- Xue, A.L.; Zhou, S.Y.; Zhao, Y.J.; Lu, X.P.; Han, P.F. Effective NH2-grafting on attapulgite surfaces for adsorption of reactive dyes. J. Hazard. Mater. 2011, 194, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, Y.; Li, M.; Zhou, S.; Xue, A.; Xing, W. Adsorption of Hg2+ from aqueous solution onto polyacrylamide/attapulgite. J. Hazard. Mater. 2009, 171, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Liu, P. Polymer modified clay minerals: A review. Appl. Clay Sci. 2007, 38, 64–76. [Google Scholar] [CrossRef]
- Manju, G.N.; Krishnan, K.A.; Vinod, V.P.; Anirudhan, T.S. An investigation into the sorption of heavy metals from wastewaters by polyacrylamide-grafted iron(III) oxide. J. Hazard. Mater. 2002, 91, 221–238. [Google Scholar] [CrossRef]
- Bicak, N.; Sherrington, D.C.; Senkal, B.F. Graft copolymer of acrylamide onto cellulose as mercury selective sorbent. React. Funct. Polym. 1999, 41, 69–76. [Google Scholar] [CrossRef]
- Liu, P.; Guo, J.S. Polyacrylamide grafted attapulgite (PAM-ATP) via surface-initiated atom transfer radical polymerization (SI-ATRP) for removal of Hg(II) ion and dyes. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282, 498–503. [Google Scholar] [CrossRef]
- Sayin, S.; Ozcan, F.; Yilmaz, M.; Tor, A.; Memon, S.; Cengeloglu, Y. Synthesis of Calix 4 arene-grafted Magnetite Nanoparticles and Evaluation of Their Arsenate as Well as Dichromate Removal Efficiency. Clean Soil Air Water 2010, 38, 639–648. [Google Scholar] [CrossRef]
- Kasgoz, H.; Ozgumus, S.; Orbay, M. Modified polyacrylamide hydrogels and their application in removal of heavy metal ions. Polymer 2003, 44, 1785–1793. [Google Scholar] [CrossRef]
- Ge, Y.Y.; Li, Z.L.; Kong, Y.; Song, Q.P.; Wang, K.Q. Heavy metal ions retention by bi-functionalized lignin: Synthesis, applications, and adsorption mechanisms. J. Ind. Eng. Chem. 2014, 20, 4429–4436. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Chen, Z.Y.; Ma, P.C. Effect of doubly organo-modified vermiculite on the properties of vermiculite/polystyrene nanocomposites. Appl. Clay Sci. 2013, 75–76, 74–81. [Google Scholar] [CrossRef]
- Liu, P. Preparation and characterization of conducting polyaniline/silica nanosheet composites. Curr. Opin. Solid State Mater. Sci. 2008, 12, 9–13. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Xue, A.L.; Zhao, Y.J.; Wang, Q.W.; Chen, Y.; Li, M.S.; Xing, W.H. Competitive adsorption of Hg2+, Pb2+ and Co2+ ions on polyacrylamide/attapulgite. Desalination 2011, 270, 269–274. [Google Scholar] [CrossRef]
- Wu, R.; Liu, J.; Wu, Q. Study on Preparation of Chelating Flocculant and Its Performance of Removing Cadmium. In Proceedings of the 2011 International Symposium on Water Resource and Environmental Protection (ISWREP), Xi’an, China, 20–22 May 2011. [Google Scholar]
- Tran, L.; Wu, P.X.; Zhu, Y.J.; Yang, L.; Zhu, N.W. Highly enhanced adsorption for the removal of Hg(II) from aqueous solution by Mercaptoethylamine/Mercaptopropyltrimethoxysilane functionalized vermiculites. J. Colloid Interface Sci. 2015, 445, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Liu, R.F.; Wang, A.Q. Preparation of starch-graft-poly(acrylamide)/attapulgite superabsorbent composite. J. Appl. Polym. Sci. 2005, 98, 1351–1357. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas solid systems with special reference to the determination of surface-area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Valente, J.S.; Tzompantzi, F.; Prince, J.; Cortez, J.G.H.; Gomez, R. Adsorption and photocatalytic degradation of phenol and 2,4 dichlorophenoxiacetic acid by Mg-Zn-Al layered double hydroxides. Appl. Catal. B Environ. 2009, 90, 330–338. [Google Scholar] [CrossRef]
- Jeon, C.; Holl, W.H. Chemical modification of chitosan and equilibrium study for mercury ion removal. Water Res. 2003, 37, 4770–4780. [Google Scholar] [CrossRef]
- Huang, X.; Hou, X.; Song, F.; Zhao, J.; Zhang, L. Facet-Dependent Cr(VI) Adsorption of Hematite Nanocrystals. Environ. Sci. Technol. 2016, 50, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Zhang, J.-S.; Zhang, B.; Wang, J.-H.; Zhao, Y.-F.; Liu, J.-D. Preparation and Characterization of Silane Coupling Agent Modified Halloysite for Cr(VI) Removal. Ind. Eng. Chem. Res. 2011, 50, 10246–10252. [Google Scholar] [CrossRef]
- Ye, G.; Bai, F.F.; Chen, G.J.; Wei, J.C.; Wang, J.C.; Chen, J. A novel well-ordered mesoporous organosilica specialized for highly selective recognition of Pb(II) by host-guest interactions. J. Mater. Chem. 2012, 22, 20878–20880. [Google Scholar] [CrossRef]
- Nonkumwong, J.; Ananta, S.; Srisombat, L. Effective removal of lead(II) from wastewater by amine-functionalized magnesium ferrite nanoparticles. RSC Adv. 2016, 6, 47382–47393. [Google Scholar] [CrossRef]
- Da’na, E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances Zur Theorieder Sogenannten Ad sorption Geloster Stoffe. Kungliga Svenska Vetenskapsakademiens Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. II. Liquids. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Wu, P.; Huang, Z.; Li, Y.; Yang, S.; Dang, Z.; Ruan, B.; Kang, C. Efficient inhibition of heavy metal release from mine tailings against acid rain exposure by triethylenetetramine intercalated montmorillonite (TETA-Mt). J. Hazard. Mater. 2016, 318, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Tirtom, V.N.; Dincer, A.; Becerik, S.; Aydemir, T.; Celik, A. Removal of lead (II) ions from aqueous solution by using crosslinked chitosan-clay beads. Desalination Water Treat. 2012, 39, 76–82. [Google Scholar] [CrossRef]
- Jiang, M.Q.; Wang, Q.P.; Jin, X.Y.; Chen, Z.L. Removal of Pb(II) from aqueous solution using modified and unmodified kaolinite clay. J. Hazard. Mater. 2009, 170, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.H.; Gao, Z.Q.; Liu, B.Z.; Hu, X.B.; Wei, Z.B.; Sun, C. Selective removal of lead from aqueous solutions by ethylenediamine-modified attapulgite. Chem. Eng. J. 2013, 223, 91–98. [Google Scholar] [CrossRef]
- Rafiei, H.R.; Shirvani, M.; Ogunseitan, O.A. Kinetics and thermodynamics of Pb sorption onto bentonite and poly(acrylic acid)/bentonite hybrid sorbent. Desalination Water Treat. 2016, 57, 22467–22479. [Google Scholar] [CrossRef]
- Liang, X.F.; Hou, W.G.; Xu, Y.M.; Sun, G.H.; Wang, L.; Sun, Y.; Qin, X. Sorption of lead ion by layered double hydroxide intercalated with diethylenetriaminepentaacetic acid. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 366, 50–57. [Google Scholar] [CrossRef]
- Datta, D.; Uslu, H. Adsorptive Separation of Lead (Pb2+) from Aqueous Solution Using Tri-n-octylamine Supported Montmorillonite. J. Chem. Eng. Data 2017, 62, 370–375. [Google Scholar] [CrossRef]
- Solener, M.; Tunali, S.; Ozcan, A.S.; Ozcan, A.; Gedikbey, T. Adsorption characteristics of lead(II) ions onto the clay/poly(methoxyethyl)acrylamide (PMEA) composite from aqueous solutions. Desalination 2008, 223, 308–322. [Google Scholar] [CrossRef]

















| Samples | Elements | a-Verm | OVerm | PAM/OVerm | g-PAM/OVerm |
|---|---|---|---|---|---|
| Elements content (weight %) | O | 58.3 | 50.9 | 55.8 | 50.8 |
| Si | 41.7 | 38.2 | 27.1 | 28.5 | |
| C | 0 | 10.9 | 13.1 | 14.8 | |
| N | 0 | 0 | 4.0 | 5.9 |
| Samples | Raw Verm | a-Verm | OVerm | PAM/OVerm | g-PAM/OVerm |
|---|---|---|---|---|---|
| SBET (m2/g) | 27.7 | 694.2 | 396.4 | 247.3 | 201.8 |
| Vtotal (m3/g) | 6.7 × 10−2 | 5.2 × 10−1 | 3.8 × 10−1 | 2.7 × 10−1 | 2.4 × 10−1 |
| Vmeso (m3/g) | 6.7 × 10−2 | 3.9 × 10−1 | 3.6 × 10−1 | 2.7 × 10−1 | 2.4 × 10−1 |
| Vmicro (m3/g) | 0 | 1.3 × 10−1 | 1.9 × 10−2 | 0 | 0 |
| Dp,a (nm) | 10.9 | 3.0 | 3.9 | 4.4 | 4.7 |
| Metal Ion | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| qe (mg·g−1) | k1 (min−1) | r2 | qe (mg·g−1) | k2 (g·mg−1·min−1) | r2 | |
| Pb2+ | 131.1 | 0.1447 | 0.9903 | 146.7 | 0.0012 | 0.9954 |
| Metal Ion | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg·g−1) | KL (L·mg−1) | r2 | KF (mg1−n·Ln·g−1) | n | r2 | |
| Pb2+ | 219.4 | 2.8 | 0.9851 | 11.3 | 1.9 | 0.9498 |
| Adsorbent | pH | T (°C) | qm (mg·g−1) | Ref. |
|---|---|---|---|---|
| Triethylene tetramine (TETA)-montmorillonite | 4.8 | 30 | 2.44 | [61] |
| Chitosan-clay composite beads | 4.5 | 25 | 7.93 | [62] |
| Modified kaolinite clay | 5.0 | 30 | 32.2 | [63] |
| EMATP-modified attapulgite | 5.0 | 30 | 158.0 | [64] |
| Poly(acrylic acid)-bentonite | 5.0 | 50 | 96.1 | [65] |
| Mg2Al-DTPA LDH | 5.5 | 30 | 170.0 | [66] |
| Montmorillonite-TOA | 7.0 | 25 | 33.1 | [67] |
| Clay/poly(methoxyethyl)acrylamide | 5.0 | 30 | 81.0 | [68] |
| g-PAM/OVerm | 5.5 | 30 | 219.4 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, S.; Wang, L.; Mao, X.; Yang, L.; Wang, C. Selective Adsorption of Pb(II) from Aqueous Solution by Triethylenetetramine-Grafted Polyacrylamide/Vermiculite. Materials 2018, 11, 514. https://doi.org/10.3390/ma11040514
Gu S, Wang L, Mao X, Yang L, Wang C. Selective Adsorption of Pb(II) from Aqueous Solution by Triethylenetetramine-Grafted Polyacrylamide/Vermiculite. Materials. 2018; 11(4):514. https://doi.org/10.3390/ma11040514
Chicago/Turabian StyleGu, Shiqing, Lan Wang, Xinyou Mao, Liping Yang, and Chuanyi Wang. 2018. "Selective Adsorption of Pb(II) from Aqueous Solution by Triethylenetetramine-Grafted Polyacrylamide/Vermiculite" Materials 11, no. 4: 514. https://doi.org/10.3390/ma11040514