Corrosion Behavior of AZ31B Magnesium Alloy Anode by Sulfate-Reducing Prokaryotes in the Tidal Flat Mud with Different Water Contents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tidal Flat Mud Conditions
2.3. Bacterium and Culture Media
2.4. Weight-Loss Determination
2.5. Surface Characterization
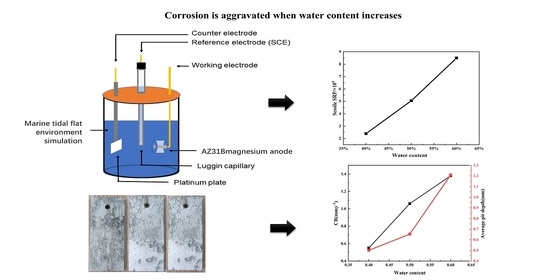
2.6. Electrochemical Corrosion Tests
3. Results
3.1. Cell Count
3.2. Surface Characterization
3.3. Weightless and Pitting Corrosion
3.4. Electrochemical Analysis
4. Discussion
5. Conclusions
- SRPs grow well in the tidal flat mud used in this experiment. The increase in the water content in mud is conducive to the growth of SRPs. Thus, the numbers of SRP increase with the increase in moisture;
- With the increase in the water content and the increase in SRPs, the corrosion process of the AZ31B magnesium anode becomes aggravated;
- The corrosion rate of the AZ31B magnesium alloy material increases with the increase in the water content of the beach soil. The loose and porous surface morphology of the corrosion products contributes to the occurrence of local corrosion.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, S.; Sun, R.; Chen, K. Self-healing performance and corrosion resistance of phytic acid/cerium composite coating on microarc-oxidized magnesium alloy. Chem. Eng. J. 2021, 428, 131198. [Google Scholar] [CrossRef]
- Cerreta, E.K.; Fensin, S.J.; Perez-Bergquist, S.J.; Trujillo, C.P.; Morrow, B.M.; Lopez, M.F.; Roach, C.J.; Mathaudhu, S.N.; Anghel, V.; Gray, G.T. The High-Strain-Rate Constitutive Behavior and Shear Response of Pure Magnesium and AZ31B Magnesium Alloy. Metall. Mater. Trans. A 2021, 52, 3152–3170. [Google Scholar] [CrossRef]
- Chaudry, U.M.; Tekumalla, S.; Gupta, M.; Jun, T.-S.; Hamad, K. Designing highly ductile magnesium alloys: Current status and future challenges. Crit. Rev. Solid State Mater. Sci. 2021, 47, 194–281. [Google Scholar] [CrossRef]
- Yan, L.; Song, G.-L.; Zheng, D. Magnesium alloy anode as a smart corrosivity detector and intelligent sacrificial anode protector for reinforced concrete. Corros. Sci. 2019, 155, 13–28. [Google Scholar] [CrossRef]
- Vanaei, H.R.; Eslami, A.; Egbewande, A. A review on pipeline corrosion, in-line inspection (ILI), and corrosion growth rate models. Int. J. Press. Vessel. Pip. 2017, 149, 43–54. [Google Scholar] [CrossRef]
- Wasim, M.; Shoaib, S.; Mubarak, N.M.; Inamuddin; Asiri, A.M. Factors influencing corrosion of metal pipes in soils. Environ. Chem. Lett. 2018, 16, 861–879. [Google Scholar] [CrossRef]
- Liu, L.; Yuan, K.; Liu, Y.; Pan, S. Microstructure and corrosion behaviour of Al2O3–13 wt-% TiO2 laser alloyed magnesium alloys. Trans. IMF 2016, 94, 99–103. [Google Scholar] [CrossRef]
- Liu, H.; Cao, F.; Song, G.-L.; Zheng, D.; Shi, Z.; Dargusch, M.S.; Atrens, A. Review of the atmospheric corrosion of magnesium alloys. J. Mater. Sci. Technol. 2019, 35, 2003–2016. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, F.; Li, Y.; Song, L.; Jiang, D.; Zeng, R.-C.; Tjong, S.C.; Chen, D.-C. Corrosion resistance of dodecanethiol-modified magnesium hydroxide coating on AZ31 magnesium alloy. Appl. Phys. A 2019, 126, 8. [Google Scholar] [CrossRef]
- Xu, L.; Liu, X.; Sun, K.; Fu, R.; Wang, G. Corrosion Behavior in Magnesium-Based Alloys for Biomedical Applications. Materials 2022, 15, 2613. [Google Scholar] [CrossRef]
- Yan, T.; Tan, L.; Zhang, B.; Yang, K. Fluoride Conversion Coating on Biodegradable AZ31B Magnesium Alloy. J. Mater. Sci. Technol. 2014, 30, 666–674. [Google Scholar] [CrossRef]
- Yan, D.; Wang, Y.; Liu, J.; Song, D.; Zhang, T.; Liu, J.; He, F.; Zhang, M.; Wang, J. Self-healing system adapted to different pH environments for active corrosion protection of magnesium alloy. J. Alloys Compd. 2020, 824, 153918. [Google Scholar] [CrossRef]
- Meng, Y.; Gao, H.; Hu, J.; Gao, L. Effect of pH value on the corrosion and corrosion fatigue behavior of AM60 magnesium alloy. J. Mater. Res. 2019, 34, 1054–1063. [Google Scholar] [CrossRef]
- Simanjuntak, S.; Cavanaugh, M.; Gandel, D.; Easton, M.; Gibson, M.; Birbilis, N. The Influence of Iron, Manganese, and Zirconium on the Corrosion of Magnesium: An Artificial Neural Network Approach. Corrosion 2015, 71, 199–208. [Google Scholar] [CrossRef]
- Pan, H.; Wang, L.; Lin, Y.; Ge, F.; Zhao, K.; Wang, X.; Cui, Z. Mechanistic study of ammonium-induced corrosion of AZ31 magnesium alloy in sulfate solution. J. Mater. Sci. Technol. 2020, 54, 1–13. [Google Scholar] [CrossRef]
- Li, Z.; Shang, Z.; Wei, X.; Zhao, Q. Corrosion resistance and cytotoxicity of AZ31 magnesium alloy with N+ ion implantation. Mater. Technol. 2019, 34, 730–736. [Google Scholar] [CrossRef]
- Khouzani, M.K.; Bahrami, A.; Hosseini-Abari, A.; Khandouzi, M.; Taheri, P. Microbiologically Influenced Corrosion of a Pipeline in a Petrochemical Plant. Metals 2019, 9, 459. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Li, L.; Qu, Q.; Kang, Y.; Zhu, B.; Yu, D.; Huang, R. Extracellular electron transfer of Bacillus cereus biofilm and its effect on the corrosion behaviour of 316L stainless steel. Colloids Surf. B Biointerfaces 2018, 173, 139–147. [Google Scholar] [CrossRef]
- Spark, A.; Wang, K.; Cole, I.; Law, D.; Ward, L. Microbiologically influenced corrosion: A review of the studies conducted on buried pipelines. Corros. Rev. 2020, 38, 231–262. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.F. Microbial corrosion of initial perforation on abandoned pipelines in wet soil containing sulfate-reducing bacteria. Colloids Surf. B Biointerfaces 2020, 190, 110899. [Google Scholar] [CrossRef]
- Chen, S.; Wang, P.; Zhang, D. Corrosion behavior of copper under biofilm of sulfate-reducing bacteria. Corros. Sci. 2014, 87, 407–415. [Google Scholar] [CrossRef]
- Gu, T.; Jia, R.; Unsal, T.; Xu, D. Toward a better understanding of microbiologically influenced corrosion caused by sulfate reducing bacteria. J. Mater. Sci. Technol. 2018, 35, 631–636. [Google Scholar] [CrossRef]
- Xu, L.; Guan, F.; Ma, Y.; Zhang, R.; Zhang, Y.; Zhai, X.; Dong, X.; Wang, Y.; Duan, J.; Hou, B. Inadequate dosing of THPS treatment increases microbially influenced corrosion of pipeline steel by inducing biofilm growth of Desulfovibrio hontreensis SY-21. Bioelectrochemistry 2022, 145, 108048. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Duan, J.; Xiao, H.; Li, Y.; Liu, H.; Guan, F.; Zhai, X. Analysis of Bacterial Community Composition of Corroded Steel Immersed in Sanya and Xiamen Seawaters in China via Method of Illumina MiSeq Sequencing. Front. Microbiol. 2017, 8, 1737. [Google Scholar] [CrossRef]
- Lin, R.; Liu, C. Surface anticorrosion methods and prospects of AZ91 series magnesium alloys. Cast. Tech. 2011, 32, 566–568. [Google Scholar]
- Liu, F.; Zhang, J.; Zhang, S.; Li, W.; Duan, J.; Hou, B. Effect of sulphate reducing bacteria on corrosion of Al-Zn-In-Sn sacrificial anodes in marine sediment. Mater. Corros. 2010, 63, 431–437. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, J.; Sun, C.; Yu, Z.; Hou, B. The corrosion of two aluminium sacrificial anode alloys in SRB-containing sea mud. Corros. Sci. 2014, 83, 375–381. [Google Scholar] [CrossRef]
- Elumalai, P.; Parthipan, P.; Narenkumar, J.; Sarankumar, R.K.; Karthikeyan, O.P.; Rajasekar, A. Influence of Thermophilic Bacteria on Corrosion of Carbon Steel in Hyper Chloride Environment. Int. J. Environ. Res. 2017, 11, 339–347. [Google Scholar] [CrossRef]
- Lan, X.; Zhang, J.; Wang, Z.; Zhang, R.; Sand, W.; Zhang, L.; Duan, J.; Zhu, Q.; Hou, B. Corrosion of an AZ31B Magnesium Alloy by Sulfate-Reducing Prokaryotes in a Mudflat Environment. Microorganisms 2022, 10, 839. [Google Scholar] [CrossRef]
- Cui, L.; Liu, Z.; Hu, P.; Shao, J. Laboratory Investigation of Microbiologically Influenced Corrosion of X80 Pipeline Steel by Sulfate-Reducing Bacteria. J. Mater. Eng. Perform. 2021, 30, 7584–7596. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, D.; Wang, P.; Cheng, Y.; Sun, S.; Sun, Y.; Chen, S. The influence of Desulfovibrio sp. and Pseudoalteromonas sp. on the corrosion of Q235 carbon steel in natural seawater. Corros. Sci. 2016, 112, 552–562. [Google Scholar] [CrossRef]
- Fu, Q.; Xu, J.; Wei, B.; Qin, Q.; Bai, Y.; Yu, C.; Sun, C. Biologically competitive effect of Desulfovibrio desulfurican and Pseudomonas stutzeri on corrosion of X80 pipeline steel in the Shenyang soil solution. Bioelectrochemistry 2022, 145, 108051. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Bu, Z.; An, Y.; Zhou, H.; Li, Y.; Chen, J. Hot corrosion product and corrosion layer evolution of La2(Zr0.75Ce0.25)2O7 coating exposed to vanadate-sulfate salts at 1050 °C. Ceram. Int. 2022, 48, 13014–13023. [Google Scholar] [CrossRef]
- Al Abbas, F.M.; Williamson, C.; Bhola, S.M.; Spear, J.R.; Olson, D.L.; Mishra, B.; Kakpovbia, A.E. Influence of sulfate reducing bacterial biofilm on corrosion behavior of low-alloy, high-strength steel (API-5L X80). Int. Biodeterior. Biodegrad. 2013, 78, 34–42. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, C.; Zhang, Y.; Liu, H. Early corrosion behavior of X80 pipeline steel in a simulated soil solution containing Desulfovibrio desulfuricans. Bioelectrochemistry 2021, 141, 107880. [Google Scholar] [CrossRef]
- Shahryari, Z.; Gheisari, K.; Motamedi, H. Effect of sulfate reducing Citrobacter sp. strain on the corrosion behavior of API X70 microalloyed pipeline steel. Mater. Chem. Phys. 2019, 236, 121799. [Google Scholar] [CrossRef]
- Guan, F.; Duan, J.; Zhai, X.; Wang, N.; Zhang, J.; Lu, D.; Hou, B. Interaction between sulfate-reducing bacteria and aluminum alloys—Corrosion mechanisms of 5052 and Al-Zn-In-Cd aluminum alloys. J. Mater. Sci. Technol. 2020, 36, 55–64. [Google Scholar] [CrossRef]
- Dou, W.; Liu, J.; Cai, W.; Wang, D.; Jia, R.; Chen, S.; Gu, T. Electrochemical investigation of increased carbon steel corrosion via extracellular electron transfer by a sulfate reducing bacterium under carbon source starvation. Corros. Sci. 2019, 150, 258–267. [Google Scholar] [CrossRef]
- Trif, L.; Shaban, A.; Telegdi, J. Electrochemical and surface analytical techniques applied to microbiologically influenced corrosion investigation. Corros. Rev. 2018, 36, 349–363. [Google Scholar] [CrossRef] [Green Version]
- Moradi, M.; Ghiara, G.; Spotorno, R.; Xu, D.; Cristiani, P. Understanding biofilm impact on electrochemical impedance spectroscopy analyses in microbial corrosion and microbial corrosion inhibition phenomena. Electrochim. Acta 2022, 426, 140803. [Google Scholar] [CrossRef]
- Yin, L.; Xu, D.; Yang, C.; Xi, T.; Chen, X.; Yang, K. Ce addition enhances the microbially induced corrosion resistance of Cu-bearing 2205 duplex stainless steel in presence of sulfate reducing bacteria. Corros. Sci. 2020, 179, 109141. [Google Scholar] [CrossRef]
- Unsal, T.; Ilhan-Sungur, E.; Arkan, S.; Cansever, N. Effects of Ag and Cu ions on the microbial corrosion of 316L stainless steel in the presence of Desulfovibrio sp. Bioelectrochemistry 2016, 110, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Zhang, D.; Lv, D.; Wang, P. Effects of two main metabolites of sulphate-reducing bacteria on the corrosion of Q235 steels in 3.5wt.% NaCl media. Corros. Sci. 2012, 65, 405–413. [Google Scholar] [CrossRef]
- Sani, F.M. Evaluation of the Simultaneous Effects of Sulfate Reducing Bacteria, Soil Type and Moisture Content on Corrosion Behavior of Buried Carbon Steel API 5L X65. Int. J. Electrochem. Sci. 2016, 11, 3887–3907. [Google Scholar] [CrossRef]
- Akkouche, R.; Rémazeilles, C.; Jeannin, M.; Barbalat, M.; Sabot, R.; Refait, P. Influence of soil moisture on the corrosion processes of carbon steel in artificial soil: Active area and differential aeration cells. Electrochim. Acta 2016, 213, 698–708. [Google Scholar] [CrossRef]
- Quej-Ake, L.; Marín-Cruz, J.; Contreras, A. Electrochemical study of the corrosion rate of API steels in clay soils. Anti Corros. Methods Mater. 2017, 64, 61–68. [Google Scholar] [CrossRef]
- Sun, B.; Liao, W.; Li, Z.; Liu, Z.; Du, C. Corrosion behavior of X65 pipeline steel in coastal areas. Anti Corros. Methods Mater. 2019, 66, 286–293. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.F. Mechanism of microbiologically influenced corrosion of X52 pipeline steel in a wet soil containing sulfate-reduced bacteria. Electrochim. Acta 2017, 253, 368–378. [Google Scholar] [CrossRef]
- Wei, B.; Xu, J.; Fu, Q.; Qin, Q.; Bai, Y.; Sun, C.; Wang, C.; Wang, Z.; Ke, W. Effect of sulfate-reducing bacteria on corrosion of X80 pipeline steel under disbonded coating in a red soil solution. J. Mater. Sci. Technol. 2021, 87, 1–17. [Google Scholar] [CrossRef]
- Cordruwisch, R.; Widdel, F. Corroding Iron as a hydrogen source for sulfate reduction in growing cultures of sulfate-reducing bacteria. Appl. Microbiol. Biotechnol. 1986, 25, 169–174. [Google Scholar] [CrossRef]
- Souissi, N.; Triki, E. Early stages of copper corrosion behaviour in a Tunisian soil. Mater. Corros. 2009, 61, 695–701. [Google Scholar] [CrossRef]
- Hirata, R.; Ooi, A.; Tada, E.; Nishikata, A. Influence of the degree of saturation on carbon steel corrosion in soil. Corros. Sci. 2021, 189, 109568. [Google Scholar] [CrossRef]
- Ding, D.; Zhang, Y.; Yu, X.; Fang, B.; Guo, J.; Li, J.; Liu, L.; Du, C. Effects of environmental factors on corrosion behavior of high-silicon cast iron in Shanxi soil medium. Anti Corros. Methods Mater. 2018, 65, 538–546. [Google Scholar] [CrossRef]
- Ezuber, H.M.; Alshater, A.; Hossain, S.M.Z.; El-Basir, A. Impact of Soil Characteristics and Moisture Content on the Corrosion of Underground Steel Pipelines. Arab. J. Sci. Eng. 2020, 46, 6177–6188. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.F. Microbial corrosion of X52 pipeline steel under soil with varied thicknesses soaked with a simulated soil solution containing sulfate-reducing bacteria and the associated galvanic coupling effect. Electrochim. Acta 2018, 266, 312–325. [Google Scholar] [CrossRef]








| Alloying Elements | Impurity Elements (≤) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mg | Al | Zn | Mn | Fe | Cu | Ni | Si | Ca |
| Bal | 2.5~3.5 | 0.60~1.40 | 0.20~1.0 | 0.003 | 0.01 | 0.001 | 0.08 | 0.04 |
| KH2PO4 | 0.25 g |
| NH4Cl | 0.5 g |
| CaCl2·6H2O | 0.03 g |
| MgSO4·7H2O | 0.06 g |
| 70% sodium lactate | 3 mL |
| Yeast Extract | 0.5 g |
| Na3C6H5O7 (trisodium citrate) | 0.15 g |
| aged seawater | 500 mL |
| Water Content | Sessile SRP Count (Cells/cm2) |
|---|---|
| 40% | (2.40 ± 0.01) × 104 |
| 50% | (5.50 ± 0.01) × 104 |
| 60% | (8.05 ± 0.01) × 104 |
| Rsol (Ω cm2) | Qf (Ω−1 cm−2sn) | nf | Rf (Ω cm2) | Qdl (Ω−1 cm−2sn) | ndl | Rct (Ω cm2) | Lpit (H·cm2) | |
|---|---|---|---|---|---|---|---|---|
| 1 d | 47.87 | 2.79 10−5 | 0.80 | 2003 | 5.71 10−4 | 0.80 | 1559 | \ |
| 3 d | 30.88 | 3.74 10−5 | 0.88 | 1469 | 2.63 10−4 | 0.94 | 2066 | 304.4 |
| 5 d | 27.54 | 1.23 10−5 | 0.95 | 37.36 | 1.26 10−5 | 0.94 | 1274 | 1104 |
| 7 d | 31.88 | 2.01 10−5 | 0.84 | 44.92 | 1.73 10−5 | 0.85 | 896.2 | 126.1 |
| 9 d | 32.98 | 1.37 10−5 | 0.84 | 167 | 2.61 10−5 | 0.82 | 1637 | 288.3 |
| 11 d | 31.06 | 5.92 10−5 | 0.79 | 987.1 | 5.07 10−5 | 0.77 | 1893 | 138.7 |
| 13 d | 22.5 | 3.38 10−5 | 0.94 | 15.71 | 3.33 10−5 | 0.93 | 1783 | 161.7 |
| Rsol (Ω cm2) | Qf (Ω−1 cm−2sn) | nf | Rf (Ω cm2) | Qdl (Ω−1 cm−2sn) | ndl | Rct (Ω cm2) | Lpit (H·cm2) | |
|---|---|---|---|---|---|---|---|---|
| 1 d | 49.37 | 2.41 10−5 | 0.89 | 2980 | 1.66 10−3 | 0.97 | 1441 | \ |
| 3 d | 52.78 | 1.1 10−5 | 0.87 | 41.1 | 1.04 10−5 | 0.88 | 902.9 | \ |
| 5 d | 34.68 | 1.12 10−5 | 0.88 | 51.54 | 1.02 10−5 | 0.88 | 1267 | 2966 |
| 7 d | 33.15 | 1.03 10−5 | 0.95 | 21.89 | 1.92 10−5 | 0.94 | 1023 | 355.5 |
| 9 d | 32.58 | 1.26 10−5 | 0.89 | 18.6 | 2.60 10−5 | 0.91 | 1350 | 1150 |
| 11 d | 33.10 | 1.59 10−5 | 0.91 | 24.27 | 2.33 10−5 | 0.92 | 1470 | 328.4 |
| 13 d | 33.97 | 1.46 10−5 | 0.93 | 233.8 | 2.49 10−5 | 0.93 | 1517 | 248.5 |
| Rsol (Ω cm2) | Qf (Ω−1 cm−2sn) | nf | Rf (Ω cm2) | Qdl (Ω−1 cm−2sn) | ndl | Rct (Ω cm2) | Lpit (H·cm2) | |
|---|---|---|---|---|---|---|---|---|
| 1 d | 40.17 | 2.39 10−5 | 0.88 | 3235 | 2.76 10−3 | 0.92 | 1023 | \ |
| 3 d | 57.29 | 2.35 10−5 | 0.85 | 4673 | 2.17 10−3 | 0.88 | 1559 | \ |
| 5 d | 28.35 | 1.43 10−5 | 0.87 | 37.86 | 1.41 10−5 | 0.91 | 1228 | 74.08 |
| 7 d | 31.87 | 1.63 10−5 | 0.80 | 27.2 | 1.47 10−5 | 0.80 | 640.6 | 87.14 |
| 9 d | 32.68 | 1.82 10−5 | 0.87 | 402.7 | 1.63 10−5 | 0.87 | 1102 | 208.2 |
| 11 d | 36.17 | 1.90 10−5 | 0.87 | 37.81 | 1.91 10−5 | 0.91 | 1266 | 219.5 |
| 13 d | 33.14 | 2.07 10−5 | 0.87 | 33.3 | 2.04 10−5 | 0.90 | 1206 | 127.6 |
| Water Content (%) | icorr (Acm−2) | Ecorr (V) vs. SCE | βa (mv/Decade) | βc (mv/Decade) |
|---|---|---|---|---|
| 40 | 4.430 × 10−6 | −1.426 | 834.2 | −139.2 |
| 50 | 5.670 × 10−6 | −1.441 | 711.3 | −626.2 |
| 60 | 9.932 × 10−6 | −1.511 | 1077.1 | −158.2 |
| Experimental Material | Conditions | Parameter | Results |
|---|---|---|---|
| X52 pipeline steel | Diluted Na2SO4 solution+ soil | water content of 5:1, 5:3, 5:5 | the MIC rate of the steel is increased by the increasing water content |
| Cu–9.4Sn and Cu–7.7Sn with 1% of lead | A mixture of the Tunisian soil with distilled water. | Electrolyte soil water content | The increase of the water electrolyte content increases material corrosion rate |
| carbon steel (SM490A) | A mixture of commercial silica sand (Marutou, Japan) with a particle size of 100 μm and a 3% NaCl aqueous solution | saturation (Sr) = 30%, 50%, 70%, 80%, 90%, 100% | The carbon steel exposed in the saturated soil (Sr = 100%) showed uniform corrosion, non-uniform corrosion in the unsaturated soil (Sr < 100%); the corrosion current density kept constant in the range of 90%Sr to 50%Sr. |
| High-silicon cast iron | The soil was selected at a depth of 1 m in the grounding. | water content is 15, 20, 25 and 30 per cent | The corrosion rate is the largest when water content is 15% and the corrosion is the lightest when water content is 30%. |
| Mild steel specimens | Four different types of Libyan soil | soils with different moisture contents (in %): 10, 12.5, 14, 16.5, 20, 25, 30, 50 and 100 | Corrosion potentials were found to possess more negative values with rising soil moisture content. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, R.; Sand, W.; Zhu, Q.; Liu, X.; Duan, J.; Hou, B.; Zhang, J. Corrosion Behavior of AZ31B Magnesium Alloy Anode by Sulfate-Reducing Prokaryotes in the Tidal Flat Mud with Different Water Contents. Lubricants 2022, 10, 293. https://doi.org/10.3390/lubricants10110293
Li J, Zhang R, Sand W, Zhu Q, Liu X, Duan J, Hou B, Zhang J. Corrosion Behavior of AZ31B Magnesium Alloy Anode by Sulfate-Reducing Prokaryotes in the Tidal Flat Mud with Different Water Contents. Lubricants. 2022; 10(11):293. https://doi.org/10.3390/lubricants10110293
Chicago/Turabian StyleLi, Jinrong, Ruiyong Zhang, Wolfgang Sand, Qingjun Zhu, Xin Liu, Jizhou Duan, Baorong Hou, and Jie Zhang. 2022. "Corrosion Behavior of AZ31B Magnesium Alloy Anode by Sulfate-Reducing Prokaryotes in the Tidal Flat Mud with Different Water Contents" Lubricants 10, no. 11: 293. https://doi.org/10.3390/lubricants10110293