The Threat of Multiple Liver Carcinogens in the Population of Laos: A Review
Abstract
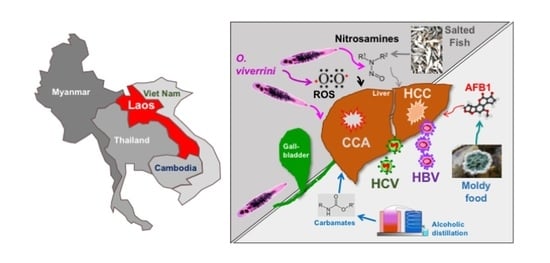
:1. Introduction
2. Methods
3. Results
3.1. Viral Risk Factors
3.1.1. Hepatitis B Virus
3.1.2. Hepatitis C Virus
3.2. Parasitic Risk Factors
3.2.1. Opisthorchiasis
3.2.2. Other Helminths
3.3. Chemical/Environmental Risk Factors
3.4. Non-Communicable Liver Diseases
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coker, R.; Hunter, B.; Rudge, J.; Liverani, M.; Hanvoravongchai, P. Emerging infectious diseases in southeast Asia: regional challenges to control. Lancet 2011, 377, 599–609. [Google Scholar] [CrossRef]
- Dans, A.; Ng, N.; Varghese, C.; Tai, E.; Firestone, R.; Bonita, R. The rise of chronic non-communicable diseases in southeast Asia: time for action. Lancet 2011, 377, 680–689. [Google Scholar] [CrossRef]
- Hong, T.; Gow, P.; Fink, M.; Dev, A.; Roberts, S.; Nicoll, A.; Lubel, J.; Kronborg, I.; Arachchi, N.; Ryan, M.; et al. Novel Population-Based Study Finding Higher Than Reported Hepatocellular Carcinoma Incidence Suggests an Updated Approach Is Needed. Hepatology 2016, 63, 1206–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Törner, A.; Stokkeland, K.; Svensson, Å.; Dickman, P.W.; Hultcrantz, R.; Montgomery, S.; Duberg, A.-S. The underreporting of hepatocellular carcinoma to the cancer register and a log-linear model to estimate a more correct incidence. Hepatology 2016, 65, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Pinyosophon, A.; Wiwanitkit, V. The Prevalence of Hepatitis B Seropositivity among Patients with Cholangiocarcinoma. Viral Immunol. 2002, 15, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Jutavijittum, P.; Yousukh, A.; Samountry, B.; Samountry, K.; Ounavong, A.; Thammavong, T.; Keokhamphue, J.; Toriyama, K. Seroprevalence of hepatitis B and C virus infections among Lao blood donors. Southeast Asian J. Trop. Med. Public Health 2007, 38, 674–679. [Google Scholar]
- Jutavijittum, P.; Andernach, I.E.; Yousukh, A.; Samountry, B.; Samountry, K.; Thammavong, T.; Keokhamphue, J.; Toriyama, K.; Muller, C.P. Occult hepatitis B infections among blood donors in Lao PDR. Vox Sang. 2014, 106, 31–37. [Google Scholar] [CrossRef]
- Black, A.P.; Nouanthong, P.; Nanthavong, N.; Souvannaso, C.; Vilivong, K.; Jutavijittum, P.; Samountry, B.; Lütteke, N.; Hübschen, J.M.; Goossens, S.; et al. Hepatitis B virus in the Lao People’s Democratic Republic: A cross sectional serosurvey in different cohorts. BMC Infect. Dis. 2014, 14, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paboriboune, P.; Vial, T.; Sitbounlang, P.; Bertani, S.; Trepo, C.; Deny, P.; Babin, F.-X.; Steenkeste, N.; Pineau, P.; Deharo, E. Hepatitis C in Laos: A 7-Year Retrospective Study on 1765 Patients. Virol. Sin. 2018, 33, 295–303. [Google Scholar] [CrossRef]
- Paboriboune, P.; Vial, T.; Chassagne, F.; Sitbounlang, P.; Soundala, S.; Bertani, S.; Sengmanothong, D.; Babin, F.-X.; Steenkeste, N.; Deny, P.; et al. A Seven-Year Retrospective Study on the Surveillance of Hepatitis B in Laos. Int. J. Hepatol. 2018, 2018, 9462475. [Google Scholar] [CrossRef] [Green Version]
- Xeuatvongsa, A.; Komada, K.; Kitamura, T.; Vongphrachanh, P.; Pathammavong, C.; Phounphenghak, K.; Sisouk, T.; Phonekeo, D.; Sengkeopaseuth, B.; Som-Oulay, V.; et al. Chronic Hepatitis B Prevalence among Children and Mothers: Results from a Nationwide, Population-Based Survey in Lao People’s Democratic Republic. PLoS ONE 2014, 9, e88829. [Google Scholar] [CrossRef] [PubMed]
- Evdokimov, K.; Sayasinh, K.; Nouanthong, P.; Vilivong, K.; Samountry, B.; Phonekeo, D.; Strobel, M.; Haegeman, F.; Heimann, P.; Muller, C.; et al. Low and disparate seroprotection after pentavalent childhood vaccination in the Lao People’s Democratic Republic: A cross-sectional study. Clin. Microbiol. Infect. 2017, 23, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Norizuki, M.; Kitamura, T.; Komada, K.; Sugiyama, M.; Mizokami, M.; Xeuatvongsa, A.; Som-Oulay, V.; Vongphrachanh, P.; Machida, M.; Wada, K.; et al. Serologic testing of randomly selected children after hepatitis B vaccination: A cross-sectional population-based study in Lao People’s Democratic Republic. BMC Infect. Dis. 2019, 19, 507. [Google Scholar] [CrossRef] [PubMed]
- Deharo, E.; Paboriboune, P.; Bourdy, G.; Elliott, E.; Rakotomalala, D.; Sengxeu, N.; Manylert, S.; Celhay, O.; Torres, J.; Lai, J.; et al. Issues, Barriers, and Facilitators that Influence Access to Immunization Programs for People Living in the Mekong Border Regions of Lao PDR; Institut de Recherche pour le Développment, University of Health Sciences, Centre d’Infectiologie Lao Christophe Mérieux: Vientiane, Laos, 2018; p. 67. [Google Scholar]
- Andernach, I.E.; Jutavijittum, P.; Samountry, B.; Yousukh, A.; Thammavong, T.; Hübschen, J.M.; Muller, C.P. A High Variability of Mixed Infections and Recent Recombinations of Hepatitis B Virus in Laos. PLoS ONE 2012, 7, e30245. [Google Scholar] [CrossRef] [PubMed]
- Bounlu, K.; Insisiengmay, S.; Vanthanouvong, K.; Widjaja, S.; Iinuma, K.; Matsubayashi, K.; Laras, K.; Putri, M.; Endy, T.; Vaughn, D.W.; et al. Acute jaundice in Vientiane, Lao People’s Democratic Republic. Clin. Infect. Dis. 1998, 27, 717–721. [Google Scholar] [CrossRef] [Green Version]
- Pybus, O.G.; Barnes, E.; Taggart, R.; Lemey, P.; Markov, P.V.; Rasachak, B.; Syhavong, B.; Phetsouvanah, R.; Sheridan, I.; Humphreys, I.S.; et al. Genetic History of Hepatitis C Virus in East Asia. J. Virol. 2008, 83, 1071–1082. [Google Scholar] [CrossRef] [Green Version]
- Hübschen, J.; Jutavijittum, P.; Thammavong, T.; Samountry, B.; Yousukh, A.; Toriyama, K.; Sausy, A.; Muller, C. High genetic diversity including potential new subtypes of hepatitis C virus genotype 6 in Lao People’s Democratic Republic. Clin. Microbiol. Infect. 2011, 17, E30–E34. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Barnes, E.; Newton, P.N.; Fu, Y.; Vongsouvath, M.; Klenerman, P.; Okamoto, H.; Abe, K.; Pybus, O.G.; Lu, L. An expanded taxonomy of hepatitis C virus genotype 6: Characterization of 22 new full-length viral genomes. Virology 2015, 476, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Hartlage, A.S.; Cullen, J.M.; Kapoor, A. The Strange, Expanding World of Animal Hepaciviruses. Annu. Rev. Virol. 2016, 3, 53–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pybus, O.G.; Thézé, J. Hepacivirus cross-species transmission and the origins of the hepatitis C virus. Curr. Opin. Virol. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Thomas, N.M.; Duckworth, J.W.; Douangboubpha, B.; Williams, M.; Francis, C.M. A Checklist of Bats (Mammalia: Chiroptera) from Lao PDR. Acta Chiropterol. 2013, 15, 193–260. [Google Scholar] [CrossRef]
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important Reservoir Hosts of Emerging Viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef] [Green Version]
- Quan, P.-L.; Firth, C.; Conte, J.M.; Williams, S.H.; Zambrana-Torrelio, C.M.; Anthony, S.J.; Ellison, J.A.; Gilbert, A.T.; Kuzmin, I.V.; Niezgoda, M.; et al. Bats are a major natural reservoir for hepaciviruses and pegiviruses. Proc. Natl. Acad. Sci. USA 2013, 110, 8194–8199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guénel, A. Lutte contre la variole en Indochine: Variolisation contre vaccination. Hist. Philos. Life Sci. 1995, 17, 55–79. [Google Scholar] [PubMed]
- Monnais-Rousselot, L. Autopsie d’un mal exotique à part: La variole et la vaccine en Indochine française (1860–1939). Rev. Française D’histoire D’outre-Mer 1995, 82, 505–527. [Google Scholar] [CrossRef]
- Sayasone, S.; Odermatt, P.; Phoumindr, N.; Vongsaravane, X.; Sensombath, V.; Phetsouvanh, R.; Choulamany, X.; Strobel, M. Epidemiology of Opisthorchis viverrini in a rural district of southern Lao PDR. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Sayasone, S.; Mak, T.K.; Vanmany, M.; Rasphone, O.; Vounatsou, P.; Utzinger, J.; Akkhavong, K.; Odermatt, P. Helminth and Intestinal Protozoa Infections, Multiparasitism and Risk Factors in Champasack Province, Lao People’s Democratic Republic. PLOS Negl. Trop. Dis. 2011, 5, e1037. [Google Scholar] [CrossRef] [Green Version]
- Sayasone, S.; Rasphone, O.; Vanmany, M.; Vounatsou, P.; Utzinger, J.; Tanner, M.; Akkhavong, K.; Hatz, C.; Odermatt, P. Severe morbidity due to Opisthorchis viverrini and Schistosoma mekongi infection in Lao People’s Democratic Republic. Clin. Inf. Dis. 2012, 55, e54–e57. [Google Scholar] [CrossRef] [Green Version]
- Soukhathammavong, P.A.; Rajpho, V.; Phongluxa, K.; Vonghachack, Y.; Hattendorf, J.; Hongvanthong, B.; Rasaphon, O.; Sripa, B.; Akkhavong, K.; Hatz, C.; et al. Subtle to severe hepatobiliary morbidity in Opisthorchis viverrini endemic settings in southern Laos. Acta Trop. 2015, 141, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Khieu, V.; Sayasone, S.; Muth, S.; Kirinoki, M.; Laymanivong, S.; Ohmae, H.; Huy, R.; Chanthapaseuth, T.; Yajima, A.; Phetsouvanh, R.; et al. Elimination of Schistosomiasis Mekongi from Endemic Areas in Cambodia and the Lao People’s Democratic Republic: Current Status and Plans. Trop. Med. Infect. Dis. 2019, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.D.; Nakamura, I.; Roberts, L.R. The tumor microenvironment in hepatocellular carcinoma: Current status and therapeutic targets. Semin. Cancer Biol. 2011, 21, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.P.; Schwank, J.; Staib, F.; Wang, X.W.; Harris, C.C. TP53 mutations and hepatocellular carcinoma: Insights into the etiology and pathogenesis of liver cancer. Oncogene 2007, 26, 2166–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedelko, T.; Arlt, V.M.; Phillips, D.H.; Hollstein, M. TP53mutation signature supports involvement of aristolochic acid in the aetiology of endemic nephropathy-associated tumours. Int. J. Cancer 2009, 124, 987–990. [Google Scholar] [CrossRef]
- Tanase, A.-M.; Marchio, A.; Dumitrascu, T.; Dima, S.; Herlea, V.; Oprisan, G.; Dejean, A.; Popescu, I.; Pineau, P. Mutation spectrum of hepatocellular carcinoma from eastern-European patients betrays the impact of a complex exposome. J. Expo. Sci. Environ. Epidemiol. 2014, 25, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, H.; Zang, M.; Wu, Q.; Zhao, H.; Lu, L.; Jiao, Y. Genetic features of aflatoxin-associated hepatocellular carcinoma. Gastroenterology 2017, 153, 249–262. [Google Scholar] [CrossRef] [Green Version]
- Hatsadong; Douangsila, K.; Gibson, P. Rice-based traditions and ritual in the Mekong River Valley. In Rice in Laos; Schiller, J., Chanphengxay, M., Linquist, B., Appa Rao, S., Eds.; International Rice Research Institute: Los Banos, Philippines, 2006; pp. 65–78. [Google Scholar]
- Delang, C. Keeping the Spirit Alive: Rice whiskey production in Northern Lao P.D.R. Ethnobot. Res. Appl. 2008, 6, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zou, H.; Fu, J.; Zhou, J.; Du, G.; Chen, J. Metabolic Engineering of the Regulators in Nitrogen Catabolite Repression to Reduce the Production of Ethyl Carbamate in a Model Rice Wine System. Appl. Environ. Microbiol. 2013, 80, 392–398. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Li, X.; Shen, C.; Lu, J.; Chen, J.; Xie, G. Decreased ethyl carbamate generation during Chinese rice wine fermentation by disruption of CAR1 in an industrial yeast strain. Int. J. Food Microbiol. 2014, 180, 19–23. [Google Scholar] [CrossRef]
- Newman, I.M.; Qian, L.; Tamrakar, N.; Zhang, B.-B. Chemical Composition and Safety of Unrecorded Grain Alcohol (Bai Jiu) Samples from Three Provinces in China. Int. J. Environ. Res. Public Health 2018, 15, 2710. [Google Scholar] [CrossRef] [Green Version]
- Mitacek, E.J.; Brunnemann, K.D.; Suttajit, M.; Martin, N.; Limsila, T.; Ohshima, H.; Caplan, L.S. Exposure to N-nitroso compounds in a population of high liver cancer regions in Thailand: Volatile nitrosamine (VNA) levels in Thai food. Food Chem. Toxicol. 1999, 37, 297–305. [Google Scholar] [CrossRef]
- Sripa, B.; Brindley, P.J.; Mulvenna, J.; Laha, T.; Smout, M.J.; Mairiang, E.; Bethony, J.M.; Loukas, A. The tumorigenic liver fluke Opisthorchis viverrini—Multiple pathways to cancer. Trends Parasitol. 2012, 28, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Srivatanakul, P.; Sukaryodhin, S.; Ohshima, H.; Khlat, M.; Parkin, M.; Brouet, I.; Bartsch, H. opisthorchis viverrini infestation and endogenous nitrosamines as risk factors for cholangiocarcinoma in thailand. Int. J. Cancer 1991, 48, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Kaewkes, S.; Sithithaworn, P.; Mairiang, E.; Laha, T.; Smout, M.; Pairojkul, C.; Bhudhisawasdi, V.; Tesana, S.; Thinkamrop, B.; et al. Liver Fluke Induces Cholangiocarcinoma. PLoS Med. 2007, 4, e201. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.C.; Kobbeman, K.; Montalbano, B.G.; Cotty, P.J. Aflatoxin-producing Aspergillus species from Thailand. Int. J. Food Microbiol. 2007, 114, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Sorn, V.; Meas, P.; Pin, T.; Gummert, M. Effects of drying and storage management on fungi (Aflatoxin B1) accumulation and rice quality in Cambodia. J. Agric. Rural Dev. Trop. Subtrop. 2017, 118, 141–148. [Google Scholar]
- Huong, B.T.M.; Tuyen, L.D.; Madsen, H.; Brimer, L.; Friis, H.; Dalsgaard, A. Total Dietary Intake and Health Risks Associated with Exposure to Aflatoxin B1, Ochratoxin A and Fuminisins of Children in Lao Cai Province, Vietnam. Toxins 2019, 11, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aye, C.N.N.; Nakagawa, H.; Kushiro, M. Occurrence of aflatoxins in processed chili pepper sold in Myanmar. JSM Mycotoxins 2019, 69, 9–13. [Google Scholar] [CrossRef]
- Galy, O.; Chemin, I.; Le Roux, E.; Villar, S.; Le Calvez-Kelm, F.; Lereau, M.; Gouas, D.; Vieco, B.; Suarez, I.; Navas, M.-C.; et al. Mutations in TP53 and CTNNB1 in Relation to Hepatitis B and C Infections in Hepatocellular Carcinomas from Thailand. Hepat. Res. Treat. 2011, 2011, 697162. [Google Scholar] [CrossRef]
- Stellman, J.M.; Stellman, S.D.; Christian, R.; Weber, T.; Tomasallo, C. The extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nat. Cell Biol. 2003, 422, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.D.; Nukaya, M.; Moran, S.M.; Glover, E.; Weinberg, S.; Balbo, S.; Hecht, S.S.; Pitot, H.C.; Drinkwater, N.R.; Bradfield, C.A. Liver Tumor Promotion by 2,3,7,8-Tetrachlorodibenzo-p-dioxin Is Dependent on the Aryl Hydrocarbon Receptor and TNF/IL-1 Receptors. Toxicol. Sci. 2014, 140, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Schecter, A.; Pavuk, M.; Päpke, O.; Ryan, J.J. Dioxin, dibenzofuran, and coplanar PCB levels in Laotian blood and milk from agent orange-sprayed and nonsprayed areas, 2001. J. Toxicol. Environ. Health Part A 2003, 66, 2067–2075. [Google Scholar] [CrossRef]
- Schecter, A.; Pavuk, M.; Malisch, R.; Ryan, J. Dioxin, Dibenzofuran, and Polychlorinated Biphenyl (PCB) Levels in Food from Agent Orange–sprayed and Nonsprayed Areas of Laos. J. Toxicol. Environ. Health Part A 2003, 66, 2165–2186. [Google Scholar] [CrossRef]
- Van Ha, M. Some peculiarities of hepatobiliary diseases in Vietnam. J. Gastroenterol. Hepatol. 1997, 12, S15–S18. [Google Scholar]
- Cordier, S.; Thuy, L.T.B.; Verger, P.; Bard, D.; Dai, L.C.; Larouzé, B.; Dazza, M.C.; Quinh, H.T.; Abenhaim, L. Viral infections and chemical exposures as risk factors for hepatocellular carcinoma in Vietnam. Int. J. Cancer 1993, 55, 196–201. [Google Scholar] [CrossRef]
- Yi, S.-W.; Ryu, S.Y.; Ohrr, H.; Hong, J.-S. Agent Orange exposure and risk of death in Korean Vietnam veterans: Korean Veterans Health Study. Int. J. Epidemiol. 2014, 43, 1825–1834. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, P.; Hazratjee, N.; Opris, D.; Agrawal, S.; Markert, R. Is exposure to Agent Orange a risk factor for hepatocellular cancer? A single-center retrospective study in the U.S. veteran population. J. Gastrointest. Oncol. 2016, 7, 426–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Wang, F.; Wong, N.-K.; He, J.; Zhang, R.; Sun, R.; Xu, Y.; Liu, Y.; Li, W.; Koike, K.; et al. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J. Hepatol. 2019, 71, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zou, B.; Yeo, Y.H.; Feng, Y.; Xie, X.; Lee, D.H.; Fujii, H.; Wu, Y.; Kam, L.Y.; Ji, F.; et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019, 4, 389–398. [Google Scholar] [CrossRef]
- Mairiang, E.; Laha, T.; Bethony, J.M.; Thinkhamrop, B.; Kaewkes, S.; Sithithaworn, P.; Tesana, S.; Loukas, A.; Brindley, P.J.; Sripa, B. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol. Int. 2012, 61, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Khuntikeo, N.; Titapun, A.; Loilome, W.; Yongvanit, P.; Thinkhamrop, B.; Chamadol, N.; Boonmars, T.; Nethanomsak, T.; Andrews, R.H.; Petney, T.N.; et al. Current Perspectives on Opisthorchiasis Control and Cholangiocarcinoma Detection in Southeast Asia. Front. Med. 2018, 5, 117. [Google Scholar] [CrossRef] [Green Version]
- Bühler, D.; Hartje, R.; Grote, U.; Dorothee, B.; Rebecca, H. Matching food security and malnutrition indicators: Evidence from Southeast Asia. Agric. Econ. 2018, 49, 481–495. [Google Scholar] [CrossRef]
- Pengpid, S.; Vonglokham, M.; Kounnavong, S.; Sychareun, V.; Peltzer, K. The prevalence of underweight and overweight/obesity and its correlates among adults in Laos: A cross-sectional national population-based survey, 2013. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2018, 25, 265–273. [Google Scholar] [CrossRef]
- Fan, J.-G.; Kim, S.-U.; Wong, V.W.-S. New trends on obesity and NAFLD in Asia. J. Hepatol. 2017, 67, 862–873. [Google Scholar] [CrossRef] [Green Version]
- Vonglokham, M.; Kounnavong, S.; Sychareun, V.; Pengpid, S.; Peltzer, K. Prevalence and social and health determinants of pre-diabetes and diabetes among adults in Laos: A cross-sectional national population-based survey, 2013. Trop. Med. Int. Health 2018, 24, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.C.S.; Huang, J.L.W.; George, J.; Huang, J.; Leung, C.; Eslam, M.; Chan, H.L.Y.; Ng, S.C. The changing epidemiology of liver diseases in the Asia–Pacific region. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Kumar, M.; Eslam, M.; George, J.; Al Mahtab, M.; Akbar, S.M.F.; Jia, J.; Tian, Q.; Aggarwal, R.; Muljono, D.H.; et al. Liver diseases in the Asia-Pacific region: A Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol. Hepatol. 2020, 5, 167–228. [Google Scholar] [CrossRef] [Green Version]
- Sornpaisarn, B.; Shield, K.; Manthey, J.; Limmade, Y.; Low, W.Y.; Van Thang, V.; Rehm, J. Alcohol consumption and attributable harm in middle-income South-East Asian countries: Epidemiology and policy options. Int. J. Drug Policy 2020, 83, 102856. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitbounlang, P.; Marchio, A.; Deharo, E.; Paboriboune, P.; Pineau, P. The Threat of Multiple Liver Carcinogens in the Population of Laos: A Review. Livers 2021, 1, 49-59. https://doi.org/10.3390/livers1010005
Sitbounlang P, Marchio A, Deharo E, Paboriboune P, Pineau P. The Threat of Multiple Liver Carcinogens in the Population of Laos: A Review. Livers. 2021; 1(1):49-59. https://doi.org/10.3390/livers1010005
Chicago/Turabian StyleSitbounlang, Philavanh, Agnès Marchio, Eric Deharo, Phimpha Paboriboune, and Pascal Pineau. 2021. "The Threat of Multiple Liver Carcinogens in the Population of Laos: A Review" Livers 1, no. 1: 49-59. https://doi.org/10.3390/livers1010005