Immune Portrayal of a New Therapy Targeting Microbiota in an Animal Model of Psoriasis
Abstract
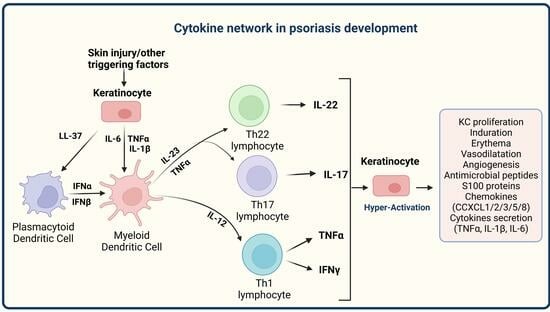
:1. Introduction
Mechanism(s) of IgY Action in the Gastrointestinal Tract
2. Materials and Methods
2.1. Isolation and Purification of IgY
2.2. IMQ-Based Murine Model
- PSO group (PSO) (8 mice—1:1 sex ratio) with induced psoriatic dermatitis as described above without treatment. The mice were sacrificed on day 7 of the experiment.
- IgY-treated PSO group (PSO IgY) (12 mice—1:1 sex ratio) with induced psoriatic dermatitis received (starting with day 7) a gavage dose of 37.5 µg IgY in a volume of 50 μL sterile PBS, for 5 consecutive days; the dose matched the dose of IgY given to a human adult (g/kg) according to a study case [29]. Mice were sacrificed on day 20 when the psoriatic lesions were macroscopically remitted.
- Purified IgY-treated PSO group (PSO PIgY) (8 mice—1:1 sex ratio) with induced psoriatic dermatitis, received the same dose of IgY but with the purified one in the same volume and were sacrificed on day 20 as above.
- Naturally remitted PSO group (Remitted PSO) (8 mice—1:1 sex ratio) with induced psoriatic dermatitis received (starting with day 7) a gavage with a volume of 50 μL sterile PBS for 5 consecutive days and then were allowed to heal naturally and sacrificed on day 22 when natural remission was assessed macroscopically.
2.3. Serum Cytokines Testing Using xMAP Array Analysis
2.4. Flow Cytometry Analysis
2.5. Statistical Analysis
3. Results
3.1. NGC-Purified IgY
3.2. IMQ-Induced Inflammation and IgY-Induced Healing
3.3. Evaluation of the Serum Cytokine/Chemokine Profile in the IgY-Treated Mice
3.3.1. IL-12 (p70) and IL-6 Circulatory Levels
3.3.2. Circulatory Levels of TNF-α and IFN-γ
3.3.3. Circulatory Levels of IL-1α and IL-1β
3.3.4. Serum Levels of IL-9, IL-15, and IL-17
3.3.5. Circulatory Levels of IL-5, IL-10, and IL-13
3.3.6. Serum Levels of MCP-1/CCL2, MIP-1α/CCL3, and MIP-1β/CCL4
3.3.7. Serum Levels of KC/CXCL1, IP-10/CXCL10, and MIG/CXCL9
3.4. Circulatory Immune Cell Populations
4. Discussion
4.1. Inflammatory Cytokines’ Improvement after IgY Treatment in Experimental PSO Model
4.2. Anti-Inflammatory Cytokines’ Improvement after IgY Treatment in the Experimental PSO Model
4.3. Inflammatory Chemokines’ Improvement after IgY Treatment in the Experimental PSO Model
4.4. Normalization of Circulatory Immune Cells after IgY Treatment in the Experimental PSO Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tashiro, T.; Sawada, Y. Psoriasis and Systemic Inflammatory Disorders. Int. J. Mol. Sci. 2022, 23, 4457. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.E.; Aschenbrenner, D.; Uhlig, H.H.; Coles, M.C.; Gaffney, E.A. A method for the inference of cytokine interaction networks. PLoS Comput. Biol. 2022, 18, e1010112. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Sung, J.H. Multiorgan-on-a-chip for realization of gut-skin axis. Biotechnol. Bioeng. 2022, 119, 2590–2601. [Google Scholar] [CrossRef] [PubMed]
- Ion, A.; Dorobanțu, A.M.; Popa, L.G.; Mihai, M.M.; Orzan, O.A. Risks of Biologic Therapy and the Importance of Multidisciplinary Approach for an Accurate Management of Patients with Moderate-Severe Psoriasis and Concomitant Diseases. Biology 2022, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Fathollahi, P.; Khedmatgozar, H.; Pourteymour Fard Tabrizi, F.; Ghareaghaj Zare, A.; Razmi, H. Probiotics Supplementation Improves Quality of Life, Clinical Symptoms, and Inflammatory Status in Patients with Psoriasis. J. Drugs Dermatol. 2022, 21, 637–644. [Google Scholar] [CrossRef]
- Wilchowski, S.M. The Role of the Gut Microbiome in Psoriasis: From Pathogens to Pathology. J. Clin. Aesthet. Dermatol. 2022, 15 (Suppl. S1), S25–S28. [Google Scholar]
- Miyagawa, F. Pathogenesis of Paradoxical Reactions Associated with Targeted Biologic Agents for Inflammatory Skin Diseases. Biomedicines 2022, 10, 1485. [Google Scholar] [CrossRef]
- Grzywa, R.; Łupicka-Słowik, A.; Sieńczyk, M. IgYs: On her majesty’s secret service. Front. Immunol. 2023, 14, 1199427. [Google Scholar] [CrossRef]
- El-Kafrawy, S.A.; Abbas, A.T.; Oelkrug, C.; Tahoon, M.; Ezzat, S.; Zumla, A.; Azhar, E.I. IgY antibodies: The promising potential to overcome antibiotic resistance. Front. Immunol. 2023, 14, 1065353. [Google Scholar] [CrossRef]
- WHO Pathogens Priority List Working Group. Discovery, research, and development of new antibiotics: The who priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Lee, L.; Samardzic, K.; Wallach, M.; Frumkin, L.R.; Mochly-Rosen, D. Immunoglobulin Y for potential diagnostic and therapeutic applications in infectious diseases. Front. Immunol. 2021, 12, 696003. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wen, Y.; Yang, F.; He, P. Chicken Egg Yolk Antibody (IgY) Protects Mice Against Enterotoxigenic Escherichia coli Infection Through Improving Intestinal Health and Immune Response. Front. Cell. Infect. Microbiol. 2021, 11, 662710. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ma, S. Anti-lipopolysaccharide egg yolk antibodies enhance the phagocytosis of mammalian phagocytes. Biol. Open 2018, 7, 032821. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.R.; Asmaa, E. Chicken Egg Yolk-IgY: Passive Immunization Promising Targeted Therapy of COVID-19 Pandemic. J. Appl. Vet. Sci. 2021, 6, 67–91. [Google Scholar]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Vieira-Pires, R.S.; Ahn, H.C.; Bok, M.; Caulfield, C.D.; Chacana, P.; Elahi, F.; Chacana, P. IgY Industries and Markets. In IgY-Technology: Production and Application of Egg Yolk Antibodies; Springer: Cham, Switzerland, 2021; pp. 279–308. [Google Scholar] [CrossRef]
- Rahman, S.; Van Nguyen, S.; Icatlo, F.C., Jr.; Umeda, K.; Kodama, Y. Oral passive IgY-based immunotherapeutics: A novel solution for prevention and treatment of alimentary tract diseases. Hum. Vaccin. Immunother. 2013, 9, 1039. [Google Scholar] [CrossRef]
- León-Núñez, D.; Vizcaíno-López, M.F.; Escorcia, M.; Correa, D.; Pérez-Hernández, E.; Gómez-Chávez, F. IgY Antibodies as Biotherapeutics in Biomedicine. Antibodies 2022, 11, 62. [Google Scholar] [CrossRef]
- Vega, C.; Bok, M.; Saif, L.; Fernandez, F.; Parreño, V. Egg yolk IgY antibodies: A therapeutic intervention against group a rotavirus in calves. Res. Vet. Sci. 2015, 103, 1–10. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, R.; Ni, P.; Chen, S.; Duan, G. Helicobacter pylori Infection and Psoriasis: A Systematic Review and Meta-Analysis. Medicina 2019, 55, 645. [Google Scholar] [CrossRef]
- de Jesús-Gil, C.; Sans-de San Nicolàs, L.; Ruiz-Romeu, E.; Ferran, M.; Soria-Martínez, L.; García-Jiménez, I. Interplay between Humoral and CLA+ T Cell Response against Candida albicans in Psoriasis. Int. J. Mol. Sci. 2021, 22, 1519. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.J.E.; Kell, D.B.; Pretorius, E. Bacterial Dysbiosis and Translocation in Psoriasis Vulgaris. Front. Cell Infect. Microbiol. 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Surcel, M.; Munteanu, A.; Isvoranu, G.; Ibram, A.; Caruntu, C.; Constantin, C. Unconventional Therapy with IgY in a Psoriatic Mouse Model Targeting Gut Microbiome. J. Pers. Med. 2021, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Pătraşcu, I.V.; Chiurciu, V.; Chiurciu, C.; Topilescu, G. Procedure to Obtain and Use Hen Egg Immunoglobulins (IgY). OSIM Patent RO129645 A0, 2014 00156, 30 July 2014. [Google Scholar]
- Chiurciu, V.; Ibram, A.; Neagu, M.; Tanase, C.; Constantin, C.; Surcel, M.; Munteanu, A.N. Method of Purification of IgY Preparation Obtained from Hyperimmune Eggs (ROMVAC) Using the Automatic High-Performance Liquid Chromatography System and of Testing the Biological Activity of the Resulting Fractions on the Standard Cell Line CAL-27. OSIM Patent RO137295A2, 2 February 2023. [Google Scholar]
- Surcel, M.; Huică, R.I.; Munteanu, A.N.; Isvoranu, G.; Pîrvu, I.R.; Ciotaru, D. Phenotypic changes of lymphocyte populations in psoriasiform dermatitis animal model. Exp. Ther. Med. 2019, 17, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Surcel, M.; Munteanu, A.N.; Huică, R.I.; Isvoranu, G.; Pîrvu, I.R.; Constantin, C. Reinforcing involvement of NK cells in psoriasiform dermatitis animal model. Exp. Ther. Med. 2019, 18, 4956–4966. [Google Scholar] [CrossRef]
- Chiurciu, C.; Chiurciu, V.; Sima, L.; Mihai, I.; Patrascu, I.V. Production and Use of Personalized (Ovopatch) Hyperimmune Egg in the Treatment of Psoriasis. OSIM Patent RO 130965 A0, 2015 00735, 30 March 2016. [Google Scholar]
- Sigurgrimsdottir, H.; Bjornsdottir, E.O.; Eysteinsdottir, J.H.; Olafsson, J.H.; Sigurgeirsson, B.; Agnarsson, B.A. Keratinocytes secrete multiple inflammatory and immune biomarkers, which are regulated by LL-37, in a psoriasis mimicking microenvironment. Scand. J. Immunol. 2021, 94, e13096. [Google Scholar] [CrossRef]
- Martinez-Sanchez, M.E.; Huerta, L.; Alvarez-Buylla, E.R.; Villarreal Luján, C. Role of Cytokine Combinations on CD4+ T Cell Differentiation, Partial Polarization, and Plasticity: Continuous Network Modeling Approach. Front. Physiol. 2018, 9, 877. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, L.; Zhou, H.; Qi, Y. A meta-analysis of Th1 and Th2 cytokine profiles differentiating tuberculous from malignant pleural effusion. Sci. Rep. 2022, 12, 2743. [Google Scholar] [CrossRef]
- Cataldi, C.; Mari, N.L.; Lozovoy, M.A.B.; Martins, L.M.M.; Reiche, E.M.V.; Maes, M. Proinflammatory and anti-inflammatory cytokine profiles in psoriasis: Use as laboratory biomarkers and disease predictors. Inflamm. Res. 2019, 68, 557–567. [Google Scholar] [CrossRef]
- Georgescu, S.R.; Tampa, M.; Caruntu, C.; Sarbu, M.I.; Mitran, C.I.; Mitran, M.I. Advances in Understanding the Immunological Pathways in Psoriasis. Int. J. Mol. Sci. 2019, 20, 739. [Google Scholar] [CrossRef]
- Jeon, C.; Sekhon, S.; Yan, D.; Afifi, L.; Nakamura, M.; Bhutani, T. Monoclonal antibodies inhibiting IL-12, -23, and -17 for the treatment of psoriasis. Hum. Vaccin. Immunother. 2017, 13, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
- Brito-Luna, M.J.; Villanueva-Quintero, D.G.; Sandoval-Talamantes, A.K.; Fafutis-Morri, M.; Graciano-Machuca, O.; Sanchez-Hernandez, P.E. Correlation of IL-12, IL-22, and IL-23 in patients with psoriasis and metabolic syndrome. Preliminary report. Cytokine 2016, 85, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Tsuji, H.; Hashimoto, Y.; Ishida-Yamamoto, A.; Iizuka, H. Serum cytokines and growth factor levels in Japanese patients with psoriasis. Clin. Exp. Dermatol. 2010, 35, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, H.; Xu, P.; Yin, Q.; Wang, Y.; Opoku, Y.K. Ameliorative effects of a fusion protein dual targeting interleukin 17A and tumor necrosis factor α on imiquimod-induced psoriasis in mice. Biomed. Pharmacother. 2018, 108, 1425–1434. [Google Scholar] [CrossRef]
- Blauvelt, A. IL-6 Differs from TNF-α: Unpredicted Clinical Effects Caused by IL-6 Blockade in Psoriasis. J. Investig. Dermatol. 2017, 137, 541–542. [Google Scholar] [CrossRef]
- Fritz, Y.; Klenotic, P.A.; Swindell, W.R.; Yin, Z.Q.; Groft, S.G.; Zhang, L. Induction of Alternative Proinflammatory Cytokines Accounts for Sustained Psoriasiform Skin Inflammation in IL-17C+IL-6KO Mice. J. Investig. Dermatol. 2017, 137, 696–705. [Google Scholar] [CrossRef]
- Bai, F.; Zheng, W.; Dong, Y.; Wang, J.; Garstka, M.A.; Li, R. Serum levels of adipokines and cytokines in psoriasis patients: A systematic review and meta-analysis. Oncotarget 2017, 9, 1266–1278. [Google Scholar] [CrossRef]
- Muramatsu, S.; Kubo, R.; Nishida, E.; Morita, A. Serum interleukin-6 levels in response to biologic treatment in patients with psoriasis. Mod. Rheumatol. 2017, 27, 137–141. [Google Scholar] [CrossRef]
- Conrad, C.; Di Domizio, J.; Mylonas, A.; Belkhodja, C.; Demaria, O.; Navarini, A.A. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat. Commun. 2018, 9, 25. [Google Scholar] [CrossRef]
- Fischer, J.A.A.; Hueber, A.J.; Wilson, S.; Galm, M.; Baum, W.; Kitson, C. Combined inhibition of tumor necrosis factor α and interleukin-17 as a therapeutic opportunity in rheumatoid arthritis: Development and characterization of a novel bispecific antibody. Arthritis Rheumatol. 2015, 67, 51–62. [Google Scholar] [CrossRef]
- Bautista-Herrera, L.A.; De la Cruz-Mosso, U.; Morales-Zambrano, R.; Villanueva-Quintero, G.D.; Hernández-Bello, J.; Ramírez-Dueñas, M.G. Expression of MIF and TNFA in psoriatic arthritis: Relationship with Th1/Th2/Th17 cytokine profiles and clinical variables. Clin. Exp. Med. 2018, 18, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Tamilselvi, E.; Haripriya, D.; Hemamalini, M.; Pushpa, G.; Swapna, S. Association of disease severity with IL-1 levels in methotrexate-treated psoriasis patients. Scand. J. Immunol. 2013, 78, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, W.C.; Oh, J.K.; Sorrenson, B.; Shepherd, P.R. Glucose regulates expression of pro-inflammatory genes, IL-1β and IL-12, through a mechanism involving hexosamine biosynthesis pathway-dependent regulation of α-E catenin. Biosci. Rep. 2021, 41, BSR20211066. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, Q.; Wu, Z.; Chen, Y.; Xu, M.; Zhang, H.; Zhao, J.; Liu, Z.; Guan, Z.; Luo, J.; et al. IL-1β-activated mTORC2 promotes accumulation of IFN-γ+ γδ T cells by upregulating CXCR3 to restrict hepatic fibrosis. Cell Death Dis. 2022, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Schön, M.P.; Wallbrecht, K.; Gruber-Wackernagel, A.; Wang, X.J.; Wolf, P. Involvement of IL-9 in Th17-Associated Inflammation and Angiogenesis of Psoriasis. PLoS ONE 2013, 8, e51752. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Z.; Chang, C.; Lu, L.; Lau, C.S.; Lu, Q. Th9 cells and IL-9 in autoimmune disorders: Pathogenesis and therapeutic potentials. Hum. Immunol. 2017, 78, 120–128. [Google Scholar] [CrossRef]
- Midde, H.S.; Priyadarssini, M.; Rajappa, M.; Munisamy, M.; Mohan Raj, P.S.; Singh, S. Interleukin-9 serves as a key link between systemic inflammation and angiogenesis in psoriasis. Clin. Exp. Dermatol. 2021, 46, 50–57. [Google Scholar] [CrossRef]
- Villadsen, L.S.; Schuurman, J.; Beurskens, F.; Dam, T.N.; Dagnaes-Hansen, F.; Skov, L. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J. Clin. Investig. 2003, 112, 1571–1580. [Google Scholar] [CrossRef]
- Isvoranu, G.; Surcel, M.; Munteanu, A.N.; Bratu, O.G.; Ionita-Radu, F.; Neagu, M.T. Therapeutic potential of interleukin-15 in cancer (Review). Exp. Ther. Med. 2021, 22, 675. [Google Scholar] [CrossRef]
- de Alcantara, C.C.; Reiche, E.M.V.; Simão, A.N.C. Chapter Five—Cytokines in psoriasis. In: Makowski GS, editor. Advances in Clinical Chemistry. Adv. Clin. Chem. 2021, 100, 171–204. [Google Scholar]
- de Oliveira, P.S.S.; Cardoso, P.R.G.; Lima, E.V.; de Pereira, M.C.; Duarte, A.L.B.P.; Pitta, I.d.R. IL-17A, IL-22, IL-6, and IL-21 Serum Levels in Plaque-Type Psoriasis in Brazilian Patients. Mediat. Inflamm. 2015, 2015, 819149. [Google Scholar] [CrossRef] [PubMed]
- Surcel, M.; Constantin, C.; Caruntu, C.; Zurac, S.; Neagu, M. Inflammatory Cytokine Pattern Is Sex-Dependent in Mouse Cutaneous Melanoma Experimental Model. J. Immunol. Res. 2017, 2017, 9212134. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Frisancho-Kiss, S.; Rose, N.R. Sex Differences in Autoimmune Disease from a Pathological Perspective. Am. J. Pathol. 2008, 173, 600–609. [Google Scholar] [PubMed]
- Ziolkowska, M.; Koc, A.; Luszczykiewicz, G.; Ksiezopolska-Pietrzak, K.; Klimczak, E.; Chwalinska-Sadowska, H. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J. Immunol. 2000, 164, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Priyadarssini, M.; Divya Priya, D.; Indhumathi, S.; Rajappa, M.; Chandrashekar, L.; Thappa, D.M. Immunophenotyping of T cells in the peripheral circulation in psoriasis. Br. J. Biomed. Sci. 2016, 73, 174–179. [Google Scholar] [CrossRef]
- Zdanowska, N.; Kasprowicz-Furmańczyk, M.; Placek, W.; Owczarczyk-Saczonek, A. The Role of Chemokines in Psoriasis—An Overview. Medicina 2021, 57, 754. [Google Scholar] [CrossRef]
- Behfar, S.; Hassanshahi, G.; Nazari, A.; Khorramdelazad, H. A brief look at the role of monocyte chemoattractant protein-1 (CCL2) in the pathophysiology of psoriasis. Cytokine 2018, 110, 226–231. [Google Scholar] [CrossRef]
- Lembo, S.; Capasso, R.; Balato, A.; Cirillo, T.; Flora, F.; Zappia, V. MCP-1 in psoriatic patients: Effect of biological therapy. J. Dermatolog. Treat. 2014, 25, 83–86. [Google Scholar] [CrossRef]
- Li, B.; Jiang, B.; Dietz, M.J.; Smith, E.S.; Clovis, N.B.; Rao, K.M.K. Evaluation of local MCP-1 and IL-12 nanocoatings for infection prevention in open fractures. J. Orthop. Res. 2010, 28, 48–54. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Ruffilli, I.; Colaci, M.; Antonelli, A.; Ferri, C.; Fallahi, P. CXCL10 in psoriasis. Adv. Med. Sci. 2015, 60, 349–354. [Google Scholar] [CrossRef]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.-J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, X.; Shi, R.; Zhang, L.; Zhao, R.; Duan, R.; Li, J. Allicin ameliorates imiquimod-induced psoriasis-likeskin inflammation via disturbing the interaction of keratinocytes with IL-17A. Br. J. Pharmacol. 2023, 180, 628–646. [Google Scholar] [CrossRef] [PubMed]
- Vijayapoopathi, S.; Ramamoorthy, R.; Meganathan, J.; Kalaiyazhagan, A.; Bhuvarahamurthy, S.; Venugopal, B. Nutraceutical combination ameliorates imiquimod-induced psoriasis in mice. Chem. Biol. Drug Des. 2023, 180, 628–646. [Google Scholar] [CrossRef] [PubMed]
- Park, K.C.; Kim, J.; Lee, A.; Lim, J.S.; Kim, K.I. Alleviation of imiquimod-induced psoriasis-like symptoms in Rorα-deficient mouse skin. BMB Rep. 2023, 56, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, R.S.; Gudjonsson, J.E.; Ward, N.L. Mouse Models of Psoriasis: A Comprehensive Review. J. Investig. Dermatol. 2022, 142 Pt B, 884–897. [Google Scholar] [CrossRef]
- Polak, K.; Bergler-Czop, B.; Szczepanek, M.; Wojciechowska, K.; Frątczak, A.; Kiss, N. Psoriasis and Gut Microbiome-Current State of Art. Int. J. Mol. Sci. 2021, 22, 4529. [Google Scholar]
- Pinget, G.V.; Tan, J.K.; Ni, D.; Taitz, J.; Daien, C.I.; Mielle, J. Dysbiosis in imiquimod-induced psoriasis alters gut immunity and exacerbates colitis development. Cell Rep. 2022, 40, 111191. [Google Scholar]
- Yegorov, S.; Babenko, D.; Kozhakhmetov, S.; Akhmaltdinova, L.; Kadyrova, I.; Nurgozhina, A. Psoriasis is Associated with Elevated Gut IL-1α and Intestinal Microbiome Alterations. Front. Immunol. 2020, 11, 571319. [Google Scholar]
- Zhang, X.; Shi, L.; Sun, T.; Guo, K.; Geng, S. Dysbiosis of gut microbiota and its correlation with dysregulation of cytokines in psoriasis patients. BMC Microbiol. 2021, 21, 78. [Google Scholar]
- Ramani, K.; Cormack, T.; Cartwright, A.N.R.; Alami, A.; Parameswaran, P.; Abdou, M. Regulation of Peripheral Inflammation by a Non-Viable, Non-Colonizing Strain of Commensal Bacteria. Front. Immunol. 2022, 13, 768076. [Google Scholar]
- Di, T.; Zhao, J.; Wang, Y.; Han, L.; Guo, X.; Han, X. Tuhuaiyin alleviates imiquimod-induced psoriasis via inhibiting the properties of IL-17-producing cells and remodels the gut microbiota. Biomed. Pharmacother. 2021, 141, 111884. [Google Scholar]
- Okada, K.; Matsushima, Y.; Mizutani, K.; Yamanaka, K. The Role of Gut Microbiome in Psoriasis: Oral Administration of Staphylococcus aureus and Streptococcus danieliae Exacerbates Skin Inflammation of Imiquimod-Induced Psoriasis-Like Dermatitis. Int. J. Mol. Sci. 2020, 21, 3303. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Deng, Y.; Fang, Z.; Zhai, Q.; Cui, S.; Zhao, J. Potential Role of Probiotics in Ameliorating Psoriasis by Modulating Gut Microbiota in Imiquimod-Induced Psoriasis-Like Mice. Nutrients 2021, 13, 2010. [Google Scholar] [CrossRef] [PubMed]













| Peak Number | Peak 1 | Peak 2 | Peak 3 | Peak 4 |
|---|---|---|---|---|
| Concentration (µg/mL) | 38 | 125 | 39 | 28 |
| Day | Day 1–9 | Day 10 | Day 11 | Day 12 | Day 13 | Day 14 |
|---|---|---|---|---|---|---|
| p-values for the IgY-treated vs. naturally remitted PSO groups | p > 0.05 | p = 0.069 | p = 0.003 | p = 0.0019 | p = 1.2 × 10−4 | p = 1.56 × 10−4 |
| Day 16 | Day 17 | Day 18 | Day 19 | Day 20 | ||
| p = 1.1 × 10−5 | p = 1.8 × 10−6 | p = 1.1 × 10−6 | p = 1.8 × 10−6 | p = 1.6 × 10−7 |
| Cytokine/Chemokine | IL-12 (p70) | IL-6 | TNF-α | IL-1α | IL-1β |
|---|---|---|---|---|---|
| PSO PIgY p-values | 0.187 | 0.400 | 0.767 | 0.470 | 0.940 |
| PSO IgY p-values | 0.270 | 0.275 | 0.910 | 0.696 | 0.943 |
| Cytokine/chemokine | IL-9 | IL-15 | IL-5 | IL-10 | MCP-1 |
| PSO PIgY | 0.695 | 0.999 | 0.459 | 0.996 | 0.337 |
| PSO IgY | 0.169 | 0.997 | 0.505 | 0.999 | 0.487 |
| Cytokine/chemokine | MIP-1α | KC | IP-10 | MIG | |
| PSO PIgY | 0.901 | 0.301 | 0.993 | 0.142 | |
| PSO IgY | 0.835 | 0.327 | 0.933 | 0.993 | |
| Immune Cells/ Cytokines/ Chemokines | T-CD3+ | T-CD4+ | T-CD8+ | B | NK |
|---|---|---|---|---|---|
| IL-1α | NS | NS | NS | r = 0.620 | NS |
| IL-1 β | NS | r = −0.468 | r = 0.456 | r = −0.743 | NS |
| IFN-γ | r = 0.32 | NS | r = 0.332 | NS | r = 0.34 |
| IL-5 | r = −0.749 | NS | NS | NS | r = −0.584 |
| IL-6 | r = 0.552 | r = 0.557 | NS | NS | r = 0.470 |
| IL-9 | NS | r = 0.405 | r = −0.602 | NS | NS |
| IL-10 | NS | NS | NS | r = −0.491 | NS |
| IL-13 | NS | r = −0.430 | NS | r = −0.869 | NS |
| IL-17 | NS | r = 0.782 | r = 0.546 | r = −0.486 | NS |
| TFN-α | r = −0.655 | NS | NS | r = 0.524 | NS |
| MIP-1α | r = 0.407 | NS | NS | NS | r = 0.525 |
| MIP-1β | r = 0.652 | NS | NS | NS | r = 0.468 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surcel, M.; Constantin, C.; Munteanu, A.N.; Costea, D.A.; Isvoranu, G.; Codrici, E.; Popescu, I.D.; Tănase, C.; Ibram, A.; Neagu, M. Immune Portrayal of a New Therapy Targeting Microbiota in an Animal Model of Psoriasis. J. Pers. Med. 2023, 13, 1556. https://doi.org/10.3390/jpm13111556
Surcel M, Constantin C, Munteanu AN, Costea DA, Isvoranu G, Codrici E, Popescu ID, Tănase C, Ibram A, Neagu M. Immune Portrayal of a New Therapy Targeting Microbiota in an Animal Model of Psoriasis. Journal of Personalized Medicine. 2023; 13(11):1556. https://doi.org/10.3390/jpm13111556
Chicago/Turabian StyleSurcel, Mihaela, Carolina Constantin, Adriana Narcisa Munteanu, Diana Antonia Costea, Gheorghița Isvoranu, Elena Codrici, Ionela Daniela Popescu, Cristiana Tănase, Alef Ibram, and Monica Neagu. 2023. "Immune Portrayal of a New Therapy Targeting Microbiota in an Animal Model of Psoriasis" Journal of Personalized Medicine 13, no. 11: 1556. https://doi.org/10.3390/jpm13111556