Assessing the Potential Risks of Digital Therapeutics (DTX): The DTX Risk Assessment Canvas
Abstract
:1. Introduction
2. Materials and Methods
3. Results
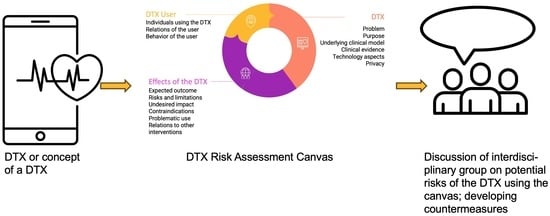
3.1. DTX Risk Assessment Canvas
3.1.1. Thematic Blocks Related to the DTX
3.1.2. Thematic Blocks Related to the User of a DTX
3.1.3. Thematic Blocks Related to the Effects of a DTX
3.1.4. Expected Use of the DTX Risk Assessment Canvas
4. Discussion
4.1. Relevance to Prior Work
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Term | Definition |
|---|---|
| Adverse event | An adverse event is an unexpected and undesirable occurrence or outcome that happens during or after a medical treatment, intervention, or the use of a DTX. Adverse events can range from mild side effects to severe complications and may include reactions to medications, medical procedures, medical device malfunctions, or incidents related to healthcare delivery. The identification, reporting, and analysis of adverse events are crucial in healthcare to monitor and improve the safety and effectiveness of treatments and interventions. |
| Clinical evidence | Clinical evidence refers to the information and data obtained from clinical research studies and trials that provide insights into the effectiveness, safety, and potential benefits or risks of medical treatments, interventions, therapies, or procedures. This evidence is gathered through systematic scientific research involving human participants under controlled conditions and is a fundamental component of evidence-based medicine. |
| Clinical model | Therapeutic approach underlying a non-digital health intervention. |
| Contraindication | A contraindication or counter-indication is a circumstance that prohibits the use of a diagnostic or therapeutic procedure in the case of a given indication or only permits it after strict consideration of the risks involved. |
| Digital Therapeutic (DTX) | DTX provide patients with evidence-based therapeutic interventions. They are delivered through high-quality software programs. |
| Gamification | Gamification is the practice of incorporating game-like elements, such as points, challenges, and rewards, into DTX to engage and motivate individuals, encouraging desired behaviors and achieving specific goals. It aims to make tasks or interactions with a DTX more enjoyable and interactive, often enhancing engagement and adherence. |
| Harm | Harm refers to any adverse effect or negative outcome experienced by individuals as a result of using DTX. This can include physical harm, such as health complications arising from the use of a medical app, as well as privacy breaches, emotional distress, or misinformation that may result from the interaction with DTX. |
| Impact | Impact in the context of DTX refers to the measurable and often intended outcomes or effects resulting from the implementation and use of DTX. These impacts can be categorized as follows: Expected Impact: These are the anticipated and planned positive outcomes that DTX aim to achieve. Expected impacts may include improved patient outcomes, enhanced access to healthcare services, increased efficiency in healthcare delivery, cost savings, and better management of health conditions. These effects are typically part of the intervention’s intended goals and objectives. Undesired impact: These are unanticipated consequences, whether positive or negative, that arise from the use of DTX. Undesired impacts can include unanticipated benefits or risks that were not initially foreseen during the development and implementation of the intervention. These effects may emerge as users engage with the technology, and they may require adjustments or further evaluation to address. |
| Problematic use | Problematic use of a DTX refers to when an individual excessively relies on or becomes overly preoccupied with a DTX or applies it for other purposes then foreseen, leading to negative impacts on their well-being or health outcomes. This can include spending too much time using the tool, prioritizing it over professional advice, experiencing negative emotions related to its use, and even neglecting other aspects of their life, potentially harming their health. |
| Privacy | Privacy refers to the protection of individuals’ personal health information and data collected, processed, or shared through DTX. It involves ensuring that sensitive health-related data are kept confidential and secure. |
| Risk | Risk refers to the potential for adverse outcomes or harm associated with the use or deployment of DTX. These risks can include issues related to data security and privacy, inaccurate health information, user dependence, and negative health consequences resulting from the intervention. |


References
- Denecke, K.B.; Elizabeth, M.; Andre, W.K. What can we learn from quality requirements in ISO/TS 82304-2 for evaluating conversational agents in healthcare? In Studies in Health Technology and Informatics; IOS Press: Amsterdam, The Netherlands, 2022; pp. 245–250. [Google Scholar]
- ISO/TS 82304-2; Technical Specification: Part 2: Health Software—Health and Wellness Apps—Quality and Reliability. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/78182.html (accessed on 20 September 2023).
- Crisafulli, S.; Santoro, E.; Recchia, G.; Trifirò, G. Digital Therapeutics in Perspective: From Regulatory Challenges to Post-Marketing Surveillance. Front. Drug Saf. Regul. 2022, 2, 900946. [Google Scholar] [CrossRef]
- Renton, T.; Tang, H.; Ennis, N.; Cusimano, M.D.; Bhalerao, S.; Schweizer, T.A.; Topolovec-Vranic, J. Web-based intervention programs for depression: A scoping review and evaluation. J. Med. Internet Res. 2014, 16, e209. [Google Scholar] [CrossRef]
- Darcy, A.; Beaudette, A.; Chiauzzi, E.; Daniels, J.; Goodwin, K.; Mariano, T.Y.; Wicks, P.; Robinson, A. Anatomy of a Woebot® (WB001): Agent guided CBT for women with postpartum depression. Expert Rev. Med. Devices 2022, 19, 287–301. [Google Scholar] [CrossRef]
- Maher, C.A.; Davis, C.R.; Curtis, R.G.; Short, C.E.; Murphy, K.J. A Physical Activity and Diet Program Delivered by Artificially Intelligent Virtual Health Coach: Proof-of-Concept Study. JMIR mHealth uHealth 2020, 8, e17558. [Google Scholar] [CrossRef] [PubMed]
- Digital Therapeutics Market Size, Share &Trends Analysis Report. Available online: https://www.grandviewresearch.com/industry-analysis/digital-therapeutics-market (accessed on 17 September 2023).
- Akili Interactive Labs. ‘EndeavorRx’. Available online: https://www.endeavorrx.com/faq/ (accessed on 5 April 2023).
- Bundesinstitut für Arzneimittel und Medizinprodukte. German Federal Institute for Drugs and Medical Devices. DiGa (Digital Health Applications). Available online: https://www.bfarm.de/EN/Medical-devices/Tasks/DiGA-and-DiPA/Digital-Health-Applications/_node.html (accessed on 20 September 2023).
- Federal Ministry of Health. Driving the Digital Transformation of Germany’s Healthcare System for the Good of Patients. Available online: https://www.bundesgesundheitsministerium.de/en/digital-healthcare-act.html (accessed on 5 April 2023).
- Bergin, A.D.G.; Valentine, A.Z.; Rennick-Egglestone, S.; Slade, M.; Hollis, C.; Hall, C.L. Identifying and Categorizing Adverse Events in Trials of Digital Mental Health Interventions: Narrative Scoping Review of Trials in the International Standard Randomized Controlled Trial Number Registry. JMIR Ment. Health 2023, 10, e42501. [Google Scholar] [CrossRef] [PubMed]
- Hering, T. Blaues Licht—Einfluss auf Schlafen und Wachen. MMW Fortschritte Med. 2020, 162, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Campos, G.; Gabarron, E.; Martin-Sanchez, F.J.; Merolli, M.; Petersen, C.; Denecke, K. Digital interventions and their unexpected outcomes—Time for digitalovigilance. In Studies in Health Technology and Informatics; IOS Press: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Osterwalder, A.; Pigneur, Y.; Clark, T. Business Model Generation: A Handbook for Visionaries, Game Changers, and Challengers; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Reijers, W.; Koidl, K.; Lewis, D.; Pandit, H.J.; Gordijn, B. Discussing ethical impacts in research and innovation: The ethics canvas. In This Changes Everything—ICT and Climate Change: What Can We Do? HCC13 2018. IFIP Advances in Information and Communication Technology; Kreps, D., Ess, C., Leenen, L., Kimppa, K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 537, pp. 299–313. [Google Scholar] [CrossRef]
- Denecke, K. Framework for Guiding the Development of High-Quality Conversational Agents in Healthcare. Healthcare 2023, 11, 1061. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, D. Understanding the dark side of gamification health management: A stress perspective. Inf. Process. Manag. 2021, 58, 102649. [Google Scholar] [CrossRef]
- Aronson, J.K.; Heneghan, C.; Ferner, R.E. Medical Devices: Definition, Classification, and Regulatory Implications. Drug Saf. 2020, 43, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.J.; Vogel, E.A.; Chieng, A.; Kendra, M.; Baiocchi, M.; Pajarito, S.; Robinson, A. A Therapeutic Relational Agent for Reducing Problematic Substance Use (Woebot): Development and Usability Study. J. Med. Internet Res. 2021, 23, e24850. [Google Scholar] [CrossRef] [PubMed]
- Medical Device Clinical Evaluation Working Group. Clinical Evidence—Key Definitions and Concepts. 2019. Available online: https://www.imdrf.org/sites/default/files/2021-09/imdrf-cons-clinical-evaluation-kdc-190405.pdf (accessed on 20 September 2023).
- May, R.; Security, K.D.; Care, I.H.S. Security, privacy, and healthcare-related conversational agents: A scoping review. Inform. Health Soc. Care 2021, 47, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Campos, G.; Merolli, M.; Martin-Sanchez, F. Biomedical Informatics and the Digital Component of the Exposome. Stud. Health Technol. Inform. 2017, 245, 496–500. [Google Scholar] [PubMed]
- Sanzari, C.M.; Gorrell, S.; Anderson, L.M.; Reilly, E.E.; Niemiec, M.A.; Orloff, N.C.; Anderson, D.A.; Hormes, J.M. The impact of social media use on body image and disordered eating behaviors: Content matters more than duration of exposure. Eat. Behav. 2023, 49, 101722. [Google Scholar] [CrossRef]
- Wang, C.; Lee, C.; Shin, H. Digital therapeutics from bench to bedside. Npj Digit. Med. 2023, 6, 38. [Google Scholar] [CrossRef]
- Huh, K.Y.; Oh, J.; Lee, S.; Yu, K.-S. Clinical Evaluation of Digital Therapeutics: Present and Future. Healthc. Inform. Res. 2022, 28, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Silvani, M.I.; Werder, R.; Perret, C. The influence of blue light on sleep, performance and wellbeing in young adults: A systematic review. Front. Physiol. 2022, 13, 943108. [Google Scholar] [CrossRef]
- Britton, W.B.; Lindahl, J.R.; Cooper, D.J.; Canby, N.K.; Palitsky, R. Defining and Measuring Meditation-Related Adverse Effects in Mindfulness-Based Programs. Clin. Psychol. Sci. 2021, 9, 1185–1204. [Google Scholar] [CrossRef] [PubMed]
- Embi, P.J. Algorithmovigilance-Advancing Methods to Analyze and Monitor Artificial Intelligence-Driven Health Care for Effectiveness and Equity. JAMA Netw. Open 2021, 4, e214622. [Google Scholar] [CrossRef] [PubMed]
- Digital Therapeutics Alliance. DTx Industry Code of Ethics. 2019. Available online: https://dtxalliance.org/wp-content/uploads/2019/11/DTA_DTx-Industry-Code-of-Ethics_11.11.19.pdf (accessed on 20 September 2023).
- ADAPT Centre & Trinity College Dublin. ‘Ethics Canvas’. 2023. Available online: https://www.ethicscanvas.org (accessed on 20 September 2023).
- O’Rourke, B.; Oortwijn, W.; Schuller, T.; International Joint Task Group. The new definition of health technology assessment: A milestone in international collaboration. Int. J. Technol. Assess. Health Care 2020, 36, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez, B.; De La Torre-Díez, I.; López-Coronado, M. Privacy and Security in Mobile Health Apps: A Review and Recommendations. J. Med. Syst. 2015, 39, 181. [Google Scholar] [CrossRef]
- Haverinen, J.; Keränen, N.; Falkenbach, P.; Maijala, A.; Kolehmainen, T.; Reponen, J. Digi-HTA: Health technology assessment framework for digital healthcare services. Finn. J. eHealth eWelfare 2019, 11, 326–341. [Google Scholar] [CrossRef]

| Guiding Questions | |
|---|---|
| DTX | |
| Problem | What is the medical condition the DTX addresses? What is it supposed to help with? |
| Purpose | What is the intended purpose of the DTX? (e.g., coaching, diagnosing, information provision, self-management support) What is the DTX expected to support, to improve, or to achieve support (e.g., having a relationship with a care provider or availability of a support person)? Is there a declared purpose as foreseen by the medical device regulation? |
| Underlying clinical model | Is the DTX modelled based on a non-digital health intervention (e.g., cognitive behavior therapy)? Which one? Which negative impacts are known for this non-digital health intervention? What is the clinical evidence of this non-digital health intervention (i.e., efficacy and safety results measured by a clinical trial)? |
| Clinical evidence | What is the underlying clinical evidence of the DTX as measured in a clinical trial? Does it differ from the clinical evidence of the related non-digital health intervention (if there is a non-digital health intervention based on which the DTX was modeled)? |
| Technology aspects that may impact outcome or the individual | What are technology aspects of the DTX that may impact the outcome of the DTX or its user (user interface design, personalization techniques, gamification, automatic adaptation, or learning…)? E.g., using gamification to increase adherence to the DTX could have a negative impact on persons with addictive behavior. |
| Privacy | Are data collected and processed by the DTX? What happens to the data? Does data storage and processing consider country-specific regulations (e.g., GDPR)? Are there any data privacy issues that could result in negative impacts on the user? |
| User of the DTX | |
| Individuals using the DTX | Who uses the DTX? (e.g., men, women, age, race, profession, health status…) Does the expected user group have specific characteristics regarding their health? |
| Relations of the user | What relations does the user have that are somehow related to the DTX? (e.g., relatives and family, healthcare professionals, social workers…). |
| Behavior of the user | How might the user’s behavior change because of the use of the DTX? How are users expected to interact with the DTX? |
| Effects of DTX | |
| Expected outcome | What is the expected outcome of the DTX? Has the outcome already been studied in a clinical trial? |
| Risks and limitations | Are there specific user groups for whom the DTX creates risks or who cannot use the DTX? Are there care settings in which the DTX should not be applied? |
| Contraindications | Are there medical conditions for which the use of the DTX should be avoided? Are there other treatments that provide a contraindication for using the DTX? |
| Undesired impact | What are the potential undesired impacts of the DTX? What happens in case of system failure? Which technology aspects might impact the outcome of the DTX (e.g., blue light can cause sleep problems)? What could go wrong? What failure could happen? How may relations of the user change through the use of the DTX? (e.g., patient–doctor relationship, family). |
| Problematic use | What could be a problematic use of the DTX? Can it be misused? |
| Relations to other interventions | What interactions with other interventions (digital or non-digital) can occur? What interactions can have an impact on the outcome of the intervention delivered through the DTX? |
| Digital Health Technology Assessment Tool | No. Domains | Domains Details | Overlapping Concepts with DTX Risk Assessment Canvas |
|---|---|---|---|
| DTX Risk Assessment Canvas | 3 | DTX description User of the DTX Effects of the DTX | |
| The Digital Technology Assessment Criteria for Health and Social Care (DTAC) (UK) 1 | 5 | Clinical Safety Data Protection Technical security Interoperability criteria Value proposition (not assessed) | Privacy, clinical evidence, functionality and purpose, and intended users |
| ORCHA Baseline review (OBR) 2 | 3 | Clinical or professional assurance Data and privacy Usability and accessibility | Privacy, clinical evidence, and functionality and purpose |
| Digi-HTA [33] | 11 | Company information Product information Technical stability Usability and accessibility Interoperability Cost Effectiveness Clinical safety Data security and protection Artificial intelligence Robotics | Privacy, clinical evidence, and functionality and purpose |
| Digital Health Assessment Framework (DHAF) (US) 3 | 4 | Data and Privacy Clinical assurance and safety Usability and accessibility Technical security and stability | Privacy, clinical evidence, and functionality and purpose |
| NorDEC (Nordic countries Europe) 4 | 5 | Data and Privacy Professional Assurance and clinical safety Usability and accessibility Security and technical stability Interoperability | Privacy, clinical evidence, and functionality and purpose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denecke, K.; May, R.; Gabarron, E.; Lopez-Campos, G.H. Assessing the Potential Risks of Digital Therapeutics (DTX): The DTX Risk Assessment Canvas. J. Pers. Med. 2023, 13, 1523. https://doi.org/10.3390/jpm13101523
Denecke K, May R, Gabarron E, Lopez-Campos GH. Assessing the Potential Risks of Digital Therapeutics (DTX): The DTX Risk Assessment Canvas. Journal of Personalized Medicine. 2023; 13(10):1523. https://doi.org/10.3390/jpm13101523
Chicago/Turabian StyleDenecke, Kerstin, Richard May, Elia Gabarron, and Guillermo H. Lopez-Campos. 2023. "Assessing the Potential Risks of Digital Therapeutics (DTX): The DTX Risk Assessment Canvas" Journal of Personalized Medicine 13, no. 10: 1523. https://doi.org/10.3390/jpm13101523