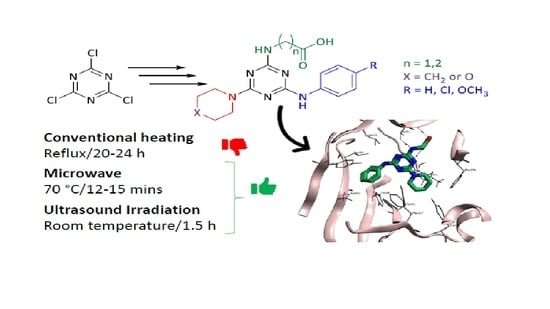
Novel 4,6-Disubstituted s-Triazin-2-yl Amino Acid Derivatives as Promising Antifungal Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedure for the Synthesis of Disubstituted Amino Acids-Triazine Derivatives
2.1.1. One-Pot Synthesis
2.1.2. Stepwise Synthesis
2.2. General Procedures for the Synthesis of 4,6,-Disubstituted s-Triazin-2-yl Amino Acid Derivatives 5a–q
2.2.1. (4-Morpholino-6-(Phenylamino)-1,3,5-Triazin-2-yl) Glycine (5a)
2.2.2. (4-((4-Chlorophenyl) Amino)-6-Morpholino-1,3,5-Triazin-2-yl) Glycine (5b)
2.2.3. (4-((4-Methoxyphenyl) Amino)-6-Morpholino-1,3,5-Triazin-2-yl) Glycine (5c)
2.2.4. (4-(Phenylamino)-6-(Piperidin-1-yl)-1,3,5-Triazin-2-yl) Glycine (5d)
2.2.5. (4-((4-Chlorophenyl) Amino)-6-(Piperidin-1-yl)-1,3,5-Triazin-2-yl) Glycine (5e)
2.2.6. (4-((4-Methoxyphenyl) Amino)-6-(Piperidin-1-yl)-1,3,5-Triazin-2-yl) Glycine (5f)
2.2.7. (4-((4-Chlorophenyl) Amino)-6-(Pyrrolidin-1-yl)-1,3,5-Triazin-2-yl) Glycine (5g)
2.2.8. (4-((4-Methoxyphenyl) Amino)-6-(Pyrrolidin-1-yl)-1,3,5-Triazin-2-yl) Glycine (5h)
2.2.9. 3-((4-Morpholino-6-(Phenylamino)-1,3,5-Triazin-2-yl) Amino) Propanoic Acid (5i)
2.2.10. 3-((4-((4-Chlorophenyl) Amino)-6-Morpholino-1,3,5-Triazin-2-yl) Amino) Propanoic Acid (5j)
2.2.11. 3-((4-((4-Methoxyphenyl) Amino)-6-Morpholino-1,3,5-Triazin-2-yl) Amino) Propanoic Acid (5k)
2.2.12. 3-((4-(Phenylamino)-6-(Piperidin-1-yl)-1,3,5-Triazin-2-yl) Amino) Propanoic Acid (5l)
2.2.13. 3-((4-((4-Chlorophenyl) Amino)-6-(Piperidin-1-yl)-1,3,5-Triazin-2-yl) Amino) Propanoic Acid (5m)
2.2.14. 3-((4-((4-Methoxyphenyl) Amino)-6-(Piperidin-1-yl)-1,3,5-Triazin-2-yl) Amino) Propanoic Acid (5n)
2.2.15. 3-((4-(Phenylamino)-6-(Pyrrolidin-1-yl)-1,3,5-Triazin-2-yl) Amino) Propanoic Acid (5o)
2.2.16. 3-((4-((4-Chlorophenyl) Amino)-6-(Pyrrolidin-1-yl)-1,3,5-Triazin-2-yl) Amino) Propanoic Acid (5p)
2.2.17. 3-((4-((4-Methoxyphenyl) Amino)-6-(Pyrrolidin-1-yl)-1,3,5-Triazin-2-yl) Amino) Propanoic Acid (5q)
2.3. Antimicrobial Activity
2.3.1. Preparation of Microbial Inoculums
2.3.2. Antimicrobial Assay Using the Disc Diffusion Method
2.3.3. Minimum Inhibitory (MIC) and Minimum Fungicidal Concentration (MFC)
2.3.4. Methodology of Docking Studies
3. Results and Discussion
3.1. Chemistry
3.2. In Vitro Antimicrobial Assays
3.3. Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blotny, G. Recent applications of 2,4,6-trichloro-1,3,5-triazine and its derivatives in organic synthesis. Tetrahedron 2006, 62, 9507–9522. [Google Scholar] [CrossRef]
- Desai, N.C.; Makwana, A.H.; Rajpara, K.M. Synthesis and study of 1,3,5-triazine based thiazole derivatives as antimicrobial agents. J. Saudi Chem. Soc. 2016, 20, S334–S341. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, K.; Srinivas, U.; Bhanuprakash, K.; Harakishore, K.; Murthy, U.S.; Rao, V.J. Synthesis and antibacterial activity of various substituted s-triazines. Eur. J. Med. Chem. 2006, 41, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.J.; Ray, C.G. Sherris Medical Microbiology; McGraw Hill: New York, NY, USA, 2004; Volume 4, ISBN 0-8385-8529-9. [Google Scholar]
- Kullberg, B.J.; Oude Lashof, A.M. Epidemiology of opportunistic invasive mycoses. Eur. J. Med. Res. 2002, 7, 183–191. [Google Scholar] [PubMed]
- Weig, M.; Groß, U.; Mühlschlegel, F. Clinical aspects and pathogenesis of Candida infection. Trends Microbiol. 1998, 6, 468–470. [Google Scholar] [CrossRef]
- Zadik, Y.; Burnstein, S.; Derazne, E.; Sandler, V.; Ianculovici, C.; Halperin, T. Colonization of Candida: Prevalence among tongue-pierced and non-pierced immunocompetent adults. Oral Dis. 2010, 16, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Achkar, J.M.; Fries, B.C. Candida infections of the genitourinary tract. Clin. Microbiol. Rev. 2010, 23, 253–273. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.T.; MacCallum, D.M.; Clancy, S.D.; Odds, F.C.; Brown, A.J.P.; Butler, G. The Candida albicans CaACE2 gene affects morphogenesis, adherence and virulence. Mol. Microbiol. 2004, 53, 969–983. [Google Scholar] [CrossRef]
- Kumamoto, C.A. Inflammation and gastrointestinal Candida colonization. Curr. Opin. Microbiol. 2011, 14, 386–391. [Google Scholar] [CrossRef] [Green Version]
- Melato, S.; Prosperi, D.; Coghi, P.; Basilico, N.; Monti, D. A Combinatorial Approach to 2,4,6-trisubstituted triazines with potent antimalarial activity: Combining conventional synthesis and microwave-assistance. Chem. Med. Chem. 2008, 3, 873–876. [Google Scholar] [CrossRef]
- Xiong, Y.-Z.; Chen, F.-E.; Balzarini, J.; De Clercq, E.; Pannecouque, C. Non-nucleoside HIV-1 reverse transcriptase inhibitors. Part 11: Structural modulations of diaryltriazines with potent anti-HIV activity. Eur. J. Med. Chem. 2008, 43, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- El-Faham, A.; Farooq, M.; Almarhoon, Z.; Alhameed, R.A.; Wadaan, M.A.M.; de la Torre, B.G.; Albericio, F. Di- and tri-substituted s-triazine derivatives: Synthesis, characterization, anticancer activity in human breast-cancer cell lines, and developmental toxicity in zebrafish embryos. Bioorg. Chem. 2020, 94, 103397. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasheed, H.H.A.; Malebari, A.M.M.; Dahlous, K.A.A.; El-Faham, A. Synthesis and Characterization of New Series of 1,3-5-Triazine Hydrazone Derivatives with Promising Antiproliferative Activity. Molecules 2020, 25, 2708. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, K.; Kumar, S.R.; Puri, S.K.; Chauhan, P.M.S. Synthesis of 9-anilinoacridine triazines as new class of hybrid antimalarial agents. Bioorg. Med. Chem. Lett. 2009, 19, 6996–6999. [Google Scholar] [CrossRef] [PubMed]
- Bhat, H.R.; Singh, U.P.; Gahtori, P.; Ghosh, S.K.; Gogoi, K.; Prakash, A.; Singh, R.K. 4-Aminoquinoline-1,3,5-triazine: Design, synthesis, in vitro antimalarial activity and docking studies. New J. Chem. 2013, 37, 2654–2662. [Google Scholar] [CrossRef]
- Pathak, P.; Thakur, A.; Bhat, H.R.; Singh, U.P. Hybrid 4-Aminoquinoline-1,3,5-triazine Derivatives: Design, Synthesis, Characterization, and Antibacterial Evaluation. J. Heterocycl. Chem. 2015, 52, 1108–1113. [Google Scholar] [CrossRef]
- Haibaa, N.S.; Khalil, H.H.; Abdel Moniem, M.; El-Wakil, M.H.; Bekhit, A.A.; Khattab, S.N. Design, synthesis and molecular modeling studies of new series of s-triazine derivatives as antimicrobial agents against multi-drug resistant clinicalisolates. Bioorg. Chem. 2019, 89, 103013. [Google Scholar] [CrossRef]
- Ramadan, D.R.; Elbardan, A.A.; Bekhit, A.A.; El-Faham, A.; Khattab, S.N. Synthesis and characterization of novel dimeric s-triazine derivatives as potential anti-bacterial agents against MDR clinical isolates. New J. Chem. 2018, 42, 10676–10688. [Google Scholar] [CrossRef]
- Sarmah, K.; Sarmah, N.; Kurmi, K.; Patel, T. Synthesis and studies of antifungal activity of 2,4,6-trisubstituted 1,3,5-triazines. Adv. Appl. Sci. Res. 2012, 3, 1459–1462. [Google Scholar]
- Saeed, S.; Rashid, N.; Jones, P.G.; Yunas, U. 2-substituted 4H-[1,3]thiazolo[3,2-a][1,3,5]triazine-4-thiones: Synthesis, crystal structure, and antifungal activity. J. Heterocycl. Chem. 2010, 47, 908–912. [Google Scholar] [CrossRef]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Khattab, S.N.; Khalil, H.H.; Bekhit, A.A.; Abd El-Rahman, M.M.; de la Torre, B.G.; El-Faham, A.; Albericio, F. 1,3,5-triazino peptide derivatives: Synthesis, characterization, and preliminary antileishmanial activity. ChemMedChem 2018, 13, 725–735. [Google Scholar] [CrossRef] [Green Version]
- Mikulová, M.B.; Kružlicová, D.; Pecher, D.; Supuran, C.T.; Mikuš, P. Synthetic Strategies and Computational Inhibition Activity Study for Triazinyl-Substituted Benzenesulfonamide Conjugates with Polar and Hydrophobic Amino Acids as Inhibitors of Carbonic Anhydrases. Int. J. Mol. Sci. 2020, 21, 3661. [Google Scholar] [CrossRef] [PubMed]
- Mikuš, P.; Krajčiová, D.; Mikulová, M.; Horváth, B.; Pecher, D.; Garaj, V.; Bua, S.; Angeli, A.; Supuran, C.T. Novel sulfonamides incorporating 1,3,5-triazine and amino acid structural motifs as inhibitors of the physiological carbonic anhydrase isozymes I, II and IV and tumor-associated isozyme IX. Bioorg. Chem. 2018, 81, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Khattab, S.N.; Khalil, H.H.; Bekhit, A.A.; El-Rahman, M.M.A.; El-Faham, A.; Albericio, F. Synthesis and preliminary biological evaluation of 1,3,5-triazine amino acid derivatives to study their MAO inhibitors. Molecules 2015, 20, 15976–15988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Exploring the Orthogonal Chemoselectivity of 2,4,6-Trichloro-1,3,5-Triazine (TCT) as a Trifunctional Linker With Different Nucleophiles: Rules of the Game. Front. Chem. 2018, 6, 516. [Google Scholar] [CrossRef] [Green Version]
- Calvete, M.J.F.; Pinto, S.M.A.; Burrows, H.D.; Castro, M.M.C.A.; Geraldes, C.F.G.C.; Pereira, M.M. Multifunctionalization of cyanuric chloride for the stepwise synthesis of potential multimodal imaging chemical entities. Arab. J. Chem. 2020, 13, 2517–2525. [Google Scholar] [CrossRef]
- Desai, N.C.; Makwana, A.H.; Senta, R.D. Synthesis, characterization and antimicrobial activity of some novel 4-(4-(arylamino)-6-(piperidin-1-yl)-1,3,5-triazine-2-ylamino)-N-(pyrimidin-2-yl)benzenesulfon amides. J. Saudi Chem. Soc. 2016, 20, 686–694. [Google Scholar] [CrossRef] [Green Version]
- Al-Marhoon, Z.; Abdel-Megeed, A.; Sholkamy, E.N.; Siddiqui, M.R.H.; El-Faham, A. Synthesis of Phenylcarbamic Acid and 2-[2-Oxo-3-(4-substituted phenylimino)-indolin-1-yl] acetohydrazide Derivatives as Promising Antifungal Agents. Asian J. Chem. 2014, 26, 7665–7672. [Google Scholar] [CrossRef]
- Wayne, P.A. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Rasadah, M.A.; Muharnad, Z. Prosid. In PerubatanTraditional Malaysia Ke-5; Universiti Malaya: Kuala Lumpur, Malaysia, 1988; p. 173. [Google Scholar]
- Toraskar, M.; Prasad, K.; Kadam, V. N-myristoyltransferase: A novel target. Mini. Rev. Med. Chem. 2008, 8, 142–149. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE), Version 2018.0101; Chemical Computing Group Inc.: Montreal, QC, Canada, 2016.
- Scheffers, D.-J.; Pinho, M.G. Bacterial Cell Wall Synthesis: New Insights from Localization Studies. Microbiol. Mol. Biol. Rev. 2005, 69, 585–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dare, K.; Ibba, M. Roles of tRNA in cell wall biosynthesis. Wiley Interdiscip. Rev. RNA 2012, 3, 247–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mewada, N.S.; Shah, D.R.; Lakum, H.P.; Chikhalia, K.H. Synthesis and biological evaluation of novel s-triazine based aryl/heteroaryl entities: Design, rationale and comparative study. J. Assoc. Arab Univ. Basic Appl. Sci. 2016, 20, 8–18. [Google Scholar] [CrossRef] [Green Version]





| Compound | Conventional (20–24 h) | Microwave (12–15 min) | Ultrasound (1.5 h) |
|---|---|---|---|
| 5a | 89 | 91 | 92 |
| 5b | 87 | 90 | 92 |
| 5c | 86 | 90 | 90 |
| 5d | 89 | 93 | 92 |
| 5e | 88 | 91 | 92 |
| 5f | 87 | 90 | 90 |
| 5g | 86 | 92 | 91 |
| 5h | 86 | 90 | 93 |
| 5i | 84 | 91 | 93 |
| 5j | 86 | 93 | 90 |
| 5k | 88 | 90 | 92 |
| 5l | 89 | 92 | 94 |
| 5m | 86 | 92 | 92 |
| 5n | 89 | 94 | 93 |
| 5o | 87 | 91 | 92 |
| 5p | 89 | 92 | 90 |
| 5q | 86 | 90 | 92 |
| Compound | C. albicans (mm) | MIC (μM) | MFC (μM) | |||
|---|---|---|---|---|---|---|
| 50 µg | 100 µg | 200 µg | 300 µg | |||
| 5a | 7 ± 0.1 | 9 ± 0.4 | 9 ± 0.2 | 10 ± 0.1 | 37.76 ± 0.7 | 75.52 ± 0.7 |
| 5b | 7 ± 0.3 | 8 ± 0.2 | 10 ± 0.1 | 12 ± 0.3 | 34.14 ± 0.1 | 68.28 ± 0.1 |
| 5c | 7 ± 0.3 | 8 ± 0.3 | 9 ± 0.3 | 10 ± 0.1 | 34.58 ± 0.8 | 69.16 ± 0.7 |
| 5d | 15 ± 0.2 | 18 ± 0.4 | 21 ± 0.3 | 23 ± 0.4 | 37.95 ± 0.2 | 75.90 ± 0.9 |
| 5e | 13 ± 0.1 | 16 ± 0.1 | 18 ± 0.3 | 20 ± 0.5 | 34.36 ± 0.6 | 68.72 ± 0.8 |
| 5f | 14 ± 0.4 | 16 ± 0.7 | 17 ± 0.4 | 19 ± 0.1 | 34.74 ± 0.3 | 69.48 ± 0.3 |
| 5g | 7 ± 0.1 | 8 ± 0.3 | 10 ± 0.3 | 11 ± 0.2 | 35.73 ± 0.7 | 71.46 ± 0.5 |
| 5h | 7 ± 0.2 | 9 ± 0.1 | 9 ± 0.3 | 10 ± 0.1 | 36.19 ± 0.2 | 72.38 ± 1.0 |
| 5i | 8 ± 0.3 | 10 ± 0.2 | 12 ± 0.4 | 12 ± 0.1 | 36.19 ± 0.4 | 72.38 ± 0.9 |
| 5j | 10 ± 0.4 | 13 ± 0.5 | 14 ± 0.3 | 15 ± 0.6 | 33.29 ± 0.3 | 66.58 ± 0.6 |
| 5k | 7 ± 0.2 | 8 ± 0.1 | 9 ± 0.3 | 10 ± 0.1 | 36.19 ± 0.2 | 72.38 ± 1.2 |
| 5l | 8 ± 0.2 | 10 ± 0.2 | 11 ± 0.3 | 12 ± 0.2 | 36.40 ± 0.3 | 72.8 ± 0.4 |
| 5m | 7 ± 0.3 | 14 ± 0.3 | 15 ± 0.2 | 15 ± 0.3 | 33.09 ± 0.1 | 66.18 ± 0.9 |
| 5n | 7 ± 0.2 | 9 ± 0.2 | 9 ± 0.1 | 10 ± 0.1 | 33.47 ± 0.5 | 66.94 ± 1.2 |
| 5o | 7 ± 0.1 | 8 ± 0.3 | 10 ± 0.2 | 10 ± 0.3 | 37.95 ± 0.7 | 75.90 ± 0.9 |
| 5p | 7 ± 0.1 | 8 ± 0.1 | 11 ± 0.3 | 11 ± 0.1 | 34.35 ± 0.4 | 68.70 ± 0.6 |
| 5q | 7 ± 0.2 | 8 ± 0.1 | 9 ± 0.4 | 10 ± 0.1 | 34.78 ± 0.6 | 69.56 ± 0.4 |
| Clotrimazole ** | - | - | - | 23 ± 0.2 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Alhameed, R.; Almarhoon, Z.; N. Sholkamy, E.; Ali Khan, S.; Ul-Haq, Z.; Sharma, A.; G. de la Torre, B.; Albericio, F.; El-Faham, A. Novel 4,6-Disubstituted s-Triazin-2-yl Amino Acid Derivatives as Promising Antifungal Agents. J. Fungi 2020, 6, 237. https://doi.org/10.3390/jof6040237
Abd Alhameed R, Almarhoon Z, N. Sholkamy E, Ali Khan S, Ul-Haq Z, Sharma A, G. de la Torre B, Albericio F, El-Faham A. Novel 4,6-Disubstituted s-Triazin-2-yl Amino Acid Derivatives as Promising Antifungal Agents. Journal of Fungi. 2020; 6(4):237. https://doi.org/10.3390/jof6040237
Chicago/Turabian StyleAbd Alhameed, Rakia, Zainab Almarhoon, Essam N. Sholkamy, Salman Ali Khan, Zaheer Ul-Haq, Anamika Sharma, Beatriz G. de la Torre, Fernando Albericio, and Ayman El-Faham. 2020. "Novel 4,6-Disubstituted s-Triazin-2-yl Amino Acid Derivatives as Promising Antifungal Agents" Journal of Fungi 6, no. 4: 237. https://doi.org/10.3390/jof6040237