Thermal Behavior of Graphene Oxide Deposited on 3D-Printed Polylactic Acid for Photothermal Therapy: An Experimental–Numerical Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. GO-PLA Manufacture: 3D Printing and Drop-Coating Procedures
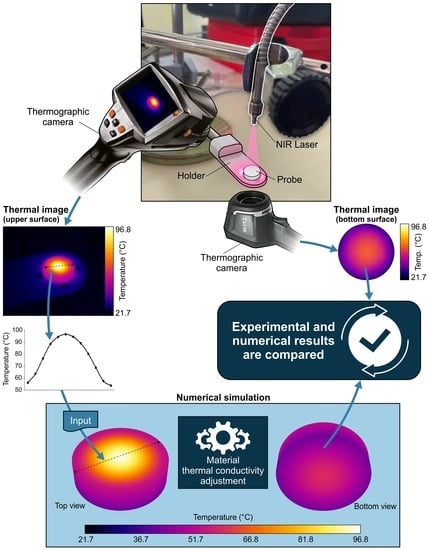
2.3. NIR Laser Source and Thermographic Cameras: Experimental Setup
- The probe was placed on the holder and both thermographic cameras captured thermal images of the initial temperature.
- The laser was switched on and the power of the laser was set to 32.73 mW. The probe was heated until a stationary state was reached. The evolution of the temperature was captured periodically with both cameras. As shown in Figure 5 for a representative case, the stabilization time was approximately 5 min.
- The laser was switched off and the probe was cooled down until it reached the initial temperature.
- The process was repeated for 93.55, 194.62, and 421.67 mW of laser power, and then repeated for all the probes of SET1 and SET2.
2.4. Numerical Analysis Methodology
- The profile of the temperature on the upper surface of the probe was extracted from the thermographic image of the stationary setup, fitted using the optimal polynomial function, and set as a boundary condition in the simulation. In this manner, the heat generation due to laser radiation was reproduced. This method was developed as an alternative to the use of Gaussian distributions [27].
- Natural convection was applied to the probe walls, using h = 10 W/m2·K and T = 20 °C, and the thermal conductivity of the material was set to an initial value.
- The simulation was conducted until it reached convergence. Then, the numerical contours of the bottom surface were extracted and compared with the corresponding thermal image. If the average temperature of the bottom surface obtained using both methods was equal, the process was finished. If not, the thermal conductivity had to be modified, and the simulation was repeated until agreement was obtained.
- The process was performed for all 40 different scenarios that were experimentally measured.
3. Results & Discussion
3.1. Thermal Characterization of the Biomaterial
3.2. Effect of the GO Mass on the Photothermal Values
3.3. The Effect of Thickness on Photothermia
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Chen, J.-L.; Yang, W.-Y.; Qian, Y.-C.; Pan, J.-Y.; Zhu, C.-N.; Liu, L.; Ou, W.-B.; Zhao, H.-X.; Zhang, D.-P. Biosynthesis of silver nanoparticles with antimicrobial and anticancer properties using two novel yeasts. Sci. Rep. 2021, 11, 15795. [Google Scholar] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom: London, UK, 2016. [Google Scholar]
- Wang, S.; Weng, J.; Fu, X.; Lin, J.; Fan, W.; Lu, N.; Qu, J.; Chen, S.; Wang, T.; Huang, P. Black Phosphorus Nanosheets for Mild Hyperthermia-Enhanced Chemotherapy and Chemo-Photothermal Combination Therapy. Nanotheranostics 2017, 1, 208–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Wang, Y.; Liu, J.; Zhai, G. Recent Developments of Phototherapy Based on Graphene Family Nanomaterials. Curr. Med. Chem. 2017, 24, 268–291. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-F.; Chang, S.-H.G.; Cheng, F.-Y.; Shanmugam, V.; Cheng, Y.-S.; Su, C.-H.; Yeh, C.-S. Au Nanorod Design as Light-Absorber in the First and Second Biological Near-Infrared Windows for In Vivo Photothermal Therapy. ACS Nano 2013, 7, 5330–5342. [Google Scholar] [CrossRef]
- Wang, D.; Kuzma, M.L.; Tan, X.; He, T.-C.; Dong, C.; Liu, Z.; Yang, J. Phototherapy and optical waveguides for the treatment of infection. Adv. Drug Deliv. Rev. 2021, 179, 114036. [Google Scholar]
- Gollavelli, G.; Ghule, A.V.; Ling, Y.-C. Multimodal Imaging and Phototherapy of Cancer and Bacterial Infection by Graphene and Related Nanocomposites. Molecules 2022, 27, 5588. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar]
- Kaushal, S.; Pinnaka, A.K.; Soni, S.; Singhal, N.K. Antibody assisted graphene oxide coated gold nanoparticles for rapid bacterial detection and near infrared light enhanced antibacterial activity. Sens. Actuators B Chem. 2021, 329, 129141. [Google Scholar] [CrossRef]
- Lei, W.; Ren, K.; Chen, T.; Chen, X.; Li, B.; Chang, H.; Ji, J. Polydopamine Nanocoating for Effective Photothermal Killing of Bacteria and Fungus upon Near-Infrared Irradiation. Adv. Mater. Interfaces 2016, 3, 1600767. [Google Scholar] [CrossRef]
- Hasan, M.T.; Lee, B.H.; Lin, C.-W.; McDonald-Boyer, A.; Gonzalez-Rodriguez, R.; Vasireddy, S.; Tsedev, U.; Coffer, J.; Belcher, A.M.; Naumov, A.V. Near-infrared emitting graphene quantum dots synthesized from reduced graphene oxide for in vitro/in vivo/ex vivo bioimaging applications. 2D Mater. 2021, 8, 035013. [Google Scholar] [CrossRef]
- Chitgupi, U.; Qin, Y.; Lovell, J.F. Targeted Nanomaterials for Phototherapy. Nanotheranostics 2017, 1, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Cheng, G.; Yu, P.; Chang, J. Light-Responsive Nanomaterials for Cancer Therapy. Engineering 2022, 13, 18–30. [Google Scholar] [CrossRef]
- Du, T.; Cao, J.; Xiao, Z.; Liu, J.; Wei, L.; Li, C.; Jiao, J.; Song, Z.; Liu, J.; Du, X.; et al. Van-mediated self-aggregating photothermal agents combined with multifunctional magnetic nickel oxide nanoparticles for precise elimination of bacterial infections. J. Nanobiotechnology 2022, 20, 325. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Li, F.; Guo, X.; Chen, W.; Dong, C.; Zhang, J.; Zhang, J.; Wang, L. Gold nanostars for cancer cell-targeted SERS-imaging and NIR light-triggered plasmonic photothermal therapy (PPTT) in the first and second biological windows. J. Mater. Chem. B 2019, 7, 2001–2008. [Google Scholar] [CrossRef]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef]
- Gai, S.; Yang, G.; Yang, P.; He, F.; Lin, J.; Jin, D.; Xing, B. Recent advances in functional nanomaterials for light–triggered cancer therapy. Nano Today 2018, 19, 146–187. [Google Scholar] [CrossRef]
- Savchuk, O.A.; Carvajal, J.J.; Massons, J.; Aguiló, M.; Díaz, F. Determination of photothermal conversion efficiency of graphene and graphene oxide through an integrating sphere method. Carbon 2016, 103, 134–141. [Google Scholar] [CrossRef]
- Ivanoska-Dacikj, A.; Bogoeva-Gaceva, G.; Krumme, A.; Tarasova, E.; Scalera, C.; Stojkovski, V.; Gjorgoski, I.; Ristoski, T. Biodegradable polyurethane/graphene oxide scaffolds for soft tissue engineering: In vivo behavior assessment. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1101–1111. [Google Scholar] [CrossRef]
- Șelaru, A.; Herman, H.; Vlăsceanu, G.M.; Dinescu, S.; Gharbia, S.; Baltă, C.; Roșu, M.; Mihali, C.V.; Ioniță, M.; Serafim, A.; et al. Graphene-Oxide Porous Biopolymer Hybrids Enhance In Vitro Osteogenic Differentiation and Promote Ectopic Osteogenesis In Vivo. Int. J. Mol. Sci. 2022, 23, 491. [Google Scholar] [CrossRef]
- Ege, D.; Kamali, A.R.; Boccaccini, A.R. Graphene Oxide/Polymer-Based Biomaterials. Adv. Eng. Mater. 2017, 19, 1700627. [Google Scholar] [CrossRef] [Green Version]
- Pérez Davila, S.; González Rodríguez, L.; Lama, R.; López-Álvarez, M.; Oliveira, A.L.; Serra, J.; Novoa, B.; Figueras, A.; González, P. 3D-Printed PLA Medical Devices: Physicochemical Changes and Biological Response after Sterilisation Treatments. Polymers 2022, 14, 4117. [Google Scholar] [CrossRef]
- Pérez Davila, S.; González Rodríguez, L.; Chiussi, S.; Serra, J.; González, P. How to sterilize polylactic acid based medical devices? Polymers 2021, 13, 2115. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-W.; Wang, S.-H.; Liu, C.-M.; Chien, M.-Y.; Hsu, C.-C.; Liu, T.-Y. Amino-modified graphene oxide nanoplatelets for photo-thermal and anti-bacterial capability. Surf. Coat. Technol. 2020, 385, 125441. [Google Scholar] [CrossRef]
- Cuiffo, M.A.; Snyder, J.; Elliott, A.M.; Romero, N.; Kannan, S.; Halada, G.P. Impact of the Fused Deposition (FDM) Printing Process on Polylactic Acid (PLA) Chemistry and Structure. Appl. Sci. 2017, 7, 579. [Google Scholar] [CrossRef] [Green Version]
- Shalwan, A.; Alajmi, A.; Yousif, B.F. Theoretical Study of the Effect of Fibre Porosity on the Heat Conductivity of Reinforced Gypsum Composite Material. Polymers 2022, 14, 3973. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Qiu, Y.; Zheng, Q.; Liu, B.; Chen, S.; Tian, Y. Finite Element Simulation of Temperature and Stress Field for Laser Cladded Nickel-Based Amorphous Composite Coatings. Coatings 2018, 8, 336. [Google Scholar] [CrossRef] [Green Version]
- Meng, C.; Zhao, J.; Yin, Y.; Luo, J.; Zhao, L.; Jiang, W.; Feng, J. Preparation and Characterization of PLA Film/3D Printing Composite Scaffold for Tissue Engineering Application. Fibers Polym. 2020, 21, 709–716. [Google Scholar] [CrossRef]
- Elkholy, A.; Rouby, M.; Kempers, R. Characterization of the anisotropic thermal conductivity of additively manufactured components by fused filament fabrication. Prog. Addit. Manuf. 2019, 4, 497–515. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Zhang, J.; Zhong, H.; Liang, J.; Huang, S.; Liao, G.; Zhang, B.; Liu, C. Fabrication and evaluation of bone morphogenetic protein-2 microspheres coated black phosphorus nanosheets@polylactic-glycolic acid copolymers scaffold: A multifunctional antibacterial photothermal scaffold for bone regeneration. Int. J. Biol. Macromol. 2022, 210, 350–364. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, J.; Ke, H.; Wang, S.; Dai, Z. Graphene oxide modified PLA microcapsules containing gold nanoparticles for ultrasonic/CT bimodal imaging guided photothermal tumor therapy. Biomaterials 2013, 34, 4794–4802. [Google Scholar] [CrossRef]











| PLA Luminy® LX175 | Value |
|---|---|
| Material density | 1.24 g/cm3 |
| Residual monomer | ≤0.3% |
| Tensile strength | 50 MPa |
| Tensile modulus | 3500 MPa |
| Melting temperature | 175 °C |
| Glass transition temperature | 60 °C |
| Tumaker Voladora NX Pellet | Parameter Values |
|---|---|
| Nozzle diameter | 0.80 mm |
| Nozzle temperature | 190–230 °C |
| Bed temperature | 45 °C |
| Infill pattern | Rectilinear (angle of 45/−45°) |
| Speed | 60 mm/s |
| Layer height | 0.20 mm |
| Probe Thickness (mm) | GO Mass (mg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SET1 | 2.85 | 0.01 | 0.02 | 0.05 | 0.10 | 0.20 | ||||
| SET2 | 1.10 | 2.20 | 2.85 | 3.40 | 5.60 | 0.10 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vence, J.; Gil, C.; González-Rodríguez, L.; López-Álvarez, M. Thermal Behavior of Graphene Oxide Deposited on 3D-Printed Polylactic Acid for Photothermal Therapy: An Experimental–Numerical Analysis. J. Funct. Biomater. 2023, 14, 80. https://doi.org/10.3390/jfb14020080
Vence J, Gil C, González-Rodríguez L, López-Álvarez M. Thermal Behavior of Graphene Oxide Deposited on 3D-Printed Polylactic Acid for Photothermal Therapy: An Experimental–Numerical Analysis. Journal of Functional Biomaterials. 2023; 14(2):80. https://doi.org/10.3390/jfb14020080
Chicago/Turabian StyleVence, Jesús, Christian Gil, Laura González-Rodríguez, and Miriam López-Álvarez. 2023. "Thermal Behavior of Graphene Oxide Deposited on 3D-Printed Polylactic Acid for Photothermal Therapy: An Experimental–Numerical Analysis" Journal of Functional Biomaterials 14, no. 2: 80. https://doi.org/10.3390/jfb14020080