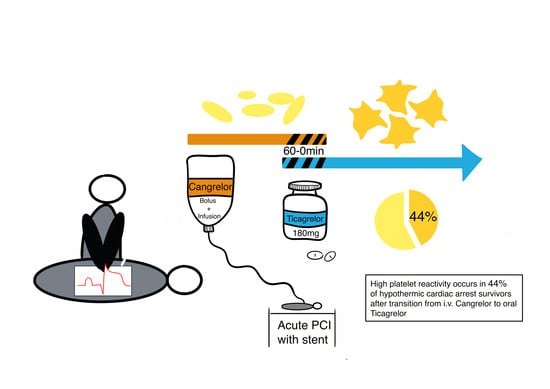
High Platelet Reactivity after Transition from Cangrelor to Ticagrelor in Hypothermic Cardiac Arrest Survivors with ST-Segment Elevation Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
Statistical Methods
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jentzer, J.C.; Scutella, M.; Pike, F.; Fitzgibbon, J.; Krehel, N.M.; Kowalski, L.; Callaway, C.W.; Rittenberger, J.C.; Reynolds, J.C.; Barsness, G.W.; et al. Early coronary angiography and percutaneous coronary intervention are associated with improved outcomes after out of hospital cardiac arrest. Resuscitation 2018, 123, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Antman, E.M.; Gibson, C.M.; Montalescot, G.; Riesmeyer, J.; Weerakkody, G.; Winters, K.J.; Warmke, J.W.; McCabe, C.H.; Braunwald, E.; et al. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: Design and rationale for the TRial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel thrombolysis in myocardial infarction 38 (TRITON-TIMI 38). Am. Heart J. 2006, 152, 627–635. [Google Scholar] [PubMed]
- Flierl, U.; Rontgen, P.; Zauner, F.; Tongers, J.; Berliner, D.; Bauersachs, J.; Schafer, A. Platelet inhibition with prasugrel in patients with acute myocardial infarction undergoing therapeutic hypothermia after cardiopulmonary resuscitation. Thromb. Haemost. 2016, 115, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.; Christoph, M.; Schmeinck, S.; Schmieder, K.; Steiding, K.; Schoener, L.; Pfluecke, C.; Quick, S.; Mues, C.; Jellinghaus, S.; et al. High rates of prasugrel and ticagrelor non-responder in patients treated with therapeutic hypothermia after cardiac arrest. Resuscitation 2014, 85, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Uminska, J.M.; Ratajczak, J.; Buszko, K.; Sobczak, P.; Sroka, W.; Marszall, M.P.; Adamski, P.; Steblovnik, K.; Noc, M.; Kubica, J. Impact of mild therapeutic hypothermia on bioavailability of ticagrelor in patients with acute myocardial infarction after out-of-hospital cardiac arrest. Cardiol. J. 2019. [Google Scholar] [CrossRef] [Green Version]
- Kubica, J.; Adamski, P.; Ostrowska, M.; Sikora, J.; Kubica, J.M.; Sroka, W.D.; Stankowska, K.; Buszko, K.; Navarese, E.P.; Jilma, B.; et al. Morphine delays and attenuates ticagrelor exposure and action in patients with myocardial infarction: The randomized, double-blind, placebo-controlled IMPRESSION trial. Eur. Heart J. 2016, 37, 245–252. [Google Scholar] [CrossRef]
- Joffre, J.; Varenne, O.; Bougouin, W.; Rosencher, J.; Mira, J.P.; Cariou, A. Stent thrombosis: An increased adverse event after angioplasty following resuscitated cardiac arrest. Resuscitation 2014, 85, 769–773. [Google Scholar] [CrossRef]
- Penela, D.; Magaldi, M.; Fontanals, J.; Martin, V.; Regueiro, A.; Ortiz, J.T.; Bosch, X.; Sabate, M.; Heras, M. Hypothermia in acute coronary syndrome: Brain salvage versus stent thrombosis? J. Am. Coll. Cardiol. 2013, 61, 686–687. [Google Scholar] [CrossRef] [Green Version]
- Majmundar, M.; Kansara, T.; Jain, A.; Shah, P.; Mithawala, P.; Desai, R.; Shah, P.; Doshi, R. Meta-analysis of the role of cangrelor for patients undergoing percutaneous coronary intervention. Am. J. Cardiol. 2019, 123, 1069–1075. [Google Scholar] [CrossRef]
- Fiore, M.; Gerbaud, E.; Coste, P.; Cetran, L.; Marchand, H.; Seguy, B. Optimal platelet inhibition with cangrelor in comatose survivors of out-of-hospital cardiac arrest undergoing primary percutaneous coronary intervention. Resuscitation 2018, 130, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Hobl, E.L.; Reiter, B.; Schoergenhofer, C.; Schwameis, M.; Derhaschnig, U.; Kubica, J.; Stimpfl, T.; Jilma, B. Morphine decreases ticagrelor concentrations but not its antiplatelet effects: A randomized trial in healthy volunteers. Eur. J. Clin. Investig. 2016, 46, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Dangas, G.D.; Schoos, M.M.; Steg, P.G.; Mehran, R.; Clemmensen, P.; van’t Hof, A.; Prats, J.; Bernstein, D.; Deliargyris, E.N.; Stone, G.W. Early stent thrombosis and mortality after primary percutaneous coronary intervention in ST-Segment-Elevation myocardial infarction: A patient-level analysis of 2 randomized trials. Circ. Cardiovasc. Interv. 2016, 9, e003272. [Google Scholar] [CrossRef] [PubMed]
- Siller-Matula, J.M.; Christ, G.; Lang, I.M.; Delle-Karth, G.; Huber, K.; Jilma, B. Multiple electrode aggregometry predicts stent thrombosis better than the vasodilator-stimulated phosphoprotein phosphorylation assay. J. Thromb. Haemost. 2010, 8, 351–359. [Google Scholar] [CrossRef]
- Siller-Matula, J.M.; Delle-Karth, G.; Lang, I.M.; Neunteufl, T.; Kozinski, M.; Kubica, J.; Maurer, G.; Linkowska, K.; Grzybowski, T.; Huber, K.; et al. Phenotyping vs. genotyping for prediction of clopidogrel efficacy and safety: The PEGASUS-PCI study. J. Thromb. Haemost. 2012, 10, 529–542. [Google Scholar] [CrossRef]
- Bonello, L.; Tantry, U.S.; Marcucci, R.; Blindt, R.; Angiolillo, D.J.; Becker, R.; Bhatt, D.L.; Cattaneo, M.; Collet, J.P.; Cuisset, T.; et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 2010, 56, 919–933. [Google Scholar] [CrossRef] [Green Version]
- Spiliopoulos, S.; Pastromas, G. Current status of high on-treatment platelet reactivity in patients with coronary or peripheral arterial disease: Mechanisms, evaluation and clinical implications. World J. Cardiol. 2015, 7, 912–921. [Google Scholar] [CrossRef]
- Garcia-Garcia, H.M.; McFadden, E.P.; Farb, A.; Mehran, R.; Stone, G.W.; Spertus, J.; Onuma, Y.; Morel, M.A.; van Es, G.A.; Zuckerman, B.; et al. Standardized end point definitions for coronary intervention trials: The academic research consortium-2 consensus document. Circulation 2018, 137, 2635–2650. [Google Scholar] [CrossRef]
- Edgren, E.; Hedstrand, U.; Kelsey, S.; Sutton-Tyrrell, K.; Safar, P. Assessment of neurological prognosis in comatose survivors of cardiac arrest. BRCT I Study Group. Lancet 1994, 343, 1055–1059. [Google Scholar] [CrossRef]
- Schneider, D.J. Transition strategies from cangrelor to oral platelet P2Y12 receptor antagonists. Coron. Artery Dis. 2016, 27, 65–69. [Google Scholar] [CrossRef]
- Schupke, S.; Neumann, F.J.; Menichelli, M.; Mayer, K.; Bernlochner, I.; Wohrle, J.; Richardt, G.; Liebetrau, C.; Witzenbichler, B.; Antoniucci, D.; et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N. Engl. J. Med. 2019, 381, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Westman, P.C.; Lipinski, M.J.; Torguson, R.; Waksman, R. A comparison of cangrelor, prasugrel, ticagrelor, and clopidogrel in patients undergoing percutaneous coronary intervention: A network meta-analysis. Cardiovasc. Revasc. Med. 2017, 18, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Franchi, F.; Rollini, F.; Rivas, A.; Wali, M.; Briceno, M.; Agarwal, M.; Shaikh, Z.; Nawaz, A.; Silva, G.; Been, L.; et al. Platelet inhibition with cangrelor and crushed ticagrelor in patients with ST-Segment-Elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation 2019, 139, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Rollini, F.; Storey, R.F.; Bhatt, D.L.; James, S.; Schneider, D.J.; Sibbing, D.; So, D.Y.F.; Trenk, D.; Alexopoulos, D.; et al. International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies. Circulation 2017, 136, 1955–1975. [Google Scholar] [CrossRef]
- Caiazzo, G.; De Rosa, S.; Torella, D.; Spaccarotella, C.; Mongiardo, A.; Giampa, S.; Micieli, M.; Palella, E.; Gulletta, E.; Indolfi, C. Administration of a loading dose has no additive effect on platelet aggregation during the switch from ongoing clopidogrel treatment to ticagrelor in patients with acute coronary syndrome. Circ. Cardiovasc. Interv. 2014, 7, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Aradi, D.; Kirtane, A.; Bonello, L.; Gurbel, P.A.; Tantry, U.S.; Huber, K.; Freynhofer, M.K.; ten Berg, J.; Janssen, P.; Angiolillo, D.J.; et al. Bleeding and stent thrombosis on P2Y12-inhibitors: Collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur. Heart J. 2015, 36, 1762–1771. [Google Scholar] [CrossRef] [Green Version]
- Aradi, D.; Storey, R.F.; Komocsi, A.; Trenk, D.; Gulba, D.; Kiss, R.G.; Husted, S.; Bonello, L.; Sibbing, D.; Collet, J.P.; et al. Expert position paper on the role of platelet function testing in patients undergoing percutaneous coronary intervention. Eur. Heart J. 2014, 35, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Tantry, U.S.; Bonello, L.; Aradi, D.; Price, M.J.; Jeong, Y.H.; Angiolillo, D.J.; Stone, G.W.; Curzen, N.; Geisler, T.; Ten Berg, J.; et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J. Am. Coll. Cardiol. 2013, 62, 2261–2273. [Google Scholar] [CrossRef]




| Variable | Total N = 16 |
|---|---|
| Male sex | 13 (81) |
| Age, years | 58 (45–61) |
| BMI, kg/m2 | 27 (25–29) |
| Comorbidities | |
| Diabetes mellitus | 2 (13) |
| Hypertension | 3 (19) |
| Smoker | 3 (19) |
| Chronic heart disease | 0 |
| Shockable rhythm | 16 (100) |
| Witnessed | 13 (81) |
| Basic life support | 13 (81) |
| Epinephrine, mg | 3 (2–4) |
| 4000IE heparin 1 | 16 (100) |
| 250 mg aspirin 1 | 16 (100) |
| Downtime 2, min | 19 (14–30) |
| Lactate, mmol/L (1.8) 3 | 3.4 (2.3–8.7) |
| Troponin T, ng/L (14) 3 | 206 (110–227) |
| Platelet count, x10E9/L (150–350) 3 | 244 (215–358) |
| NT-proBNP, pg/mL (125) 3 | 236 (126–416) |
| ASAT, U/l (17–59; 14–36) 4 | 280 (170–646) |
| ALAT, U/l (50; 35) 4 | 142 (105–241) |
| Gamma-GT, U/L (15–73; 12–43) 4 | 69 (57–122) |
| Bilirubin, mg/dL (1.20) 3 | 0.53 (0.44–0.74) |
| Blood pressure (BP), mmHg | |
| - Systolic BP | 112 (98–131) |
| - Diastolic BP | 66 (59–78) |
| - Mean BP | 81 (76–94) |
| Heart rate, bpm | 78 (48–84) |
| Temperature, °C | 33 (33–34) |
| Left ventricular systolic function | |
| - Normal | 3 (19) |
| - Mild dysfunction | 8 (50) |
| - Moderate dysfunction | 2 (13) |
| - Severe dysfunction | 3 (19) |
| Duration of cangrelor infusion, min | 147 (127–180) |
| Ticagrelor administration before cangrelor cessation, min | 39 (5–50) |
| Number of implanted coronary stents | 1 (1–2) |
| CPC 1-2 at hospital discharge | 12 (75) |
| Number of Patients (n, %) | Dose (Median, IQR) | |
|---|---|---|
| Continuous administration | ||
| Norepinephrine (µg/kg/min) | 12 (75) | 0.061 (0.050–0.129) |
| Propofol 2% (mg/kg/h) | 13 (81) | 1.33 (1.20–1.71) |
| Midazolam (µg/kg/h) | 3 (19) | 0.211 (0.171–0.217) |
| Remifentanil (µg/kg/min) | 13 (81) | 0.106 (0.090–0.118) |
| Fentanyl (µg/kg/h) | 3 (19) | 2.000 (2.000–2.053) |
| Rocuronium (mg/h) | 16 (100) | 21.75 (18.00–25.50) |
| Insulin (IU/h) | 2 (13) | 3.5 (2.75–4.25) |
| Bolus administration | ||
| Amoxicillin/Clavulanic Acid (g) | 2 (13) | 2.2 |
| Pantoprazole (mg) | 3 (19) | 40 |
| Amiodarone (mg) | 3 (19) | 300 |
| Atorvastatin (mg) | 2 (13) | 80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchtele, N.; Herkner, H.; Schörgenhofer, C.; Merrelaar, A.; Laggner, R.; Gelbenegger, G.; Spiel, A.O.; Domanovits, H.; Lang, I.; Jilma, B.; et al. High Platelet Reactivity after Transition from Cangrelor to Ticagrelor in Hypothermic Cardiac Arrest Survivors with ST-Segment Elevation Myocardial Infarction. J. Clin. Med. 2020, 9, 583. https://doi.org/10.3390/jcm9020583
Buchtele N, Herkner H, Schörgenhofer C, Merrelaar A, Laggner R, Gelbenegger G, Spiel AO, Domanovits H, Lang I, Jilma B, et al. High Platelet Reactivity after Transition from Cangrelor to Ticagrelor in Hypothermic Cardiac Arrest Survivors with ST-Segment Elevation Myocardial Infarction. Journal of Clinical Medicine. 2020; 9(2):583. https://doi.org/10.3390/jcm9020583
Chicago/Turabian StyleBuchtele, Nina, Harald Herkner, Christian Schörgenhofer, Anne Merrelaar, Roberta Laggner, Georg Gelbenegger, Alexander O. Spiel, Hans Domanovits, Irene Lang, Bernd Jilma, and et al. 2020. "High Platelet Reactivity after Transition from Cangrelor to Ticagrelor in Hypothermic Cardiac Arrest Survivors with ST-Segment Elevation Myocardial Infarction" Journal of Clinical Medicine 9, no. 2: 583. https://doi.org/10.3390/jcm9020583