Association of Pregnant Women’s Perinatal Depression with Sociodemographic, Anthropometric and Lifestyle Factors and Perinatal and Postnatal Outcomes: A Cross-Sectional Study
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Assessment of Sociodemographic and Anthropometric Factors
2.3. Assessment of Lifestyle Factors
2.4. Assessment of Perinatal and Postnatal Outcomes
2.5. Statistical Analysis
3. Results
3.1. Descriptive Statistics of the Study Population
3.2. Association of Pregnant Women’s Perinatal Depression with Sociodemographic, Anthropometric and Lifestyle Factors
3.3. Association of Pregnant Women’s Perinatal Depressive Symptoms with Perinatal and Postnatal Outcomes
3.4. Multivariate Binary Logistic Regression Analysis for Pregnant Women’s Perinatal Depression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Mental Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 7 January 2024).
- The Lancet Global Health. Mental health matters. Lancet Glob. Health 2020, 8, e1352. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Agostino, D.; Wu, Y.T.; Daskalopoulou, C.; Hasan, M.T.; Huisman, M.; Prina, M. Global trends in the prevalence and incidence of depression: A systematic review and meta-analysis. J. Affect. Disord. 2021, 281, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Soto-Fernández, I.; Gómez-Cantarino, S.; Yáñez-Araque, B.; Sánchez-Infante, J.; Zapata-Ossa, A.; Dios-Aguado, M. A Cross-Sectional Study Examining the Association between Physical Activity and Perinatal Depression. Medicina 2022, 58, 1174. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.H.F.; Lin, X.; Griva, K.; Subramaniam, M.; Ćelić, I.; Tudor Car, L. Information needs and sources of information among people with depression and anxiety: A scoping review. BMC Psychiatry 2022, 22, 502. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Potdar, J. Maternal Mental Health During Pregnancy: A Critical Review. Cureus 2022, 14, e30656. [Google Scholar] [CrossRef] [PubMed]
- Fadzil, A.; Balakrishnan, K.; Razali, R.; Sidi, H.; Malapan, T.; Japaraj, R.P.; Midin, M.; Nik Jaafar, N.R.; Das, S.; Manaf, M.R. Risk factors for depression and anxiety among pregnant women in Hospital Tuanku Bainun, Ipoh, Malaysia. Asia Pac. Psychiatry 2013, 5 (Suppl. S1), 7–13. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, R.; Dai, X.; Liu, H.; Zhang, J.; Liu, X.; Si, D.; Deng, T.; Xia, W. The association between symptoms of depression during pregnancy and low birth weight: A prospective study. BMC Pregnancy Childbirth 2020, 20, 147. [Google Scholar] [CrossRef] [PubMed]
- Pampaka, D.; Papatheodorou, S.I.; AlSeaidan, M.; Al Wotayan, R.; Wright, R.J.; Buring, J.E.; Dockery, D.W.; Christophi, C.A. Antenatal depressive symptoms and adverse perinatal outcomes. BMC Pregnancy Childbirth 2021, 21, 313. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Yu, J.J.; Zeng, W.T.; Zhou, M.C.; Duan, C.C.; Zhu, L.L. Association between antenatal depression and adverse perinatal outcomes: A prospective cohort study. J. Affect. Disord. 2023, 323, 490–495. [Google Scholar] [CrossRef]
- Ko, J.Y.; Farr, S.L.; Dietz, P.M.; Robbins, C.L. Depression and treatment among U.S. pregnant and nonpregnant women of reproductive age, 2005–2009. J. Womens Health 2012, 21, 830–836. [Google Scholar] [CrossRef]
- Oates, M. Suicide: The leading cause of maternal death. Br. J. Psychiatry 2003, 183, 279–281. [Google Scholar] [CrossRef]
- Howard, L.M.; Khalifeh, H. Perinatal mental health: A review of progress and challenges. World Psychiatry 2020, 19, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Yirmiya, K.; Yakirevich-Amir, N.; Preis, H.; Lotan, A.; Atzil, S.; Reuveni, I. Women’s Depressive Symptoms during the COVID-19 Pandemic: The Role of Pregnancy. Int. J. Environ. Res. Public Health 2021, 18, 4298. [Google Scholar] [CrossRef]
- Basu, A.; Kim, H.H.; Basaldua, R.; Choi, K.W.; Charron, L.; Kelsall, N.; Hernandez-Diaz, S.; Wyszynski, D.F.; Koenen, K.C. A cross-national study of factors associated with women’s perinatal mental health and wellbeing during the COVID-19 pandemic. PLoS ONE 2021, 16, e0249780. [Google Scholar] [CrossRef]
- Ahmad, M.; Vismara, L. The Psychological Impact of COVID-19 Pandemic on Women’s Mental Health during Pregnancy: A Rapid Evidence Review. Int. J. Environ. Res. Public Health 2021, 18, 7112. [Google Scholar] [CrossRef]
- Milgrom, J.; Hirshler, Y.; Reece, J.; Holt, C.; Gemmill, A.W. Social Support-A Protective Factor for Depressed Perinatal Women? Int. J. Environ. Res. Public Health 2019, 16, 1426. [Google Scholar] [CrossRef]
- Lantigua-Martinez, M.; Trostle, M.E.; Torres, A.M.; Rajeev, P.; Dennis, A.; Silverstein, J.S.; Talib, M. Perinatal depression before and during the COVID-19 pandemic in New York City. AJOG Glob. Rep. 2023, 3, 100253. [Google Scholar] [CrossRef]
- Lebel, C.; MacKinnon, A.; Bagshawe, M.; Tomfohr-Madsen, L.; Giesbrecht, G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J. Affect. Disord. 2020, 277, 5–13. [Google Scholar] [CrossRef]
- Aktas, S.; Yesilcicek Calik, K. Factors Affecting Depression During Pregnancy and the Correlation Between Social Support and Pregnancy Depression. Iran. Red. Crescent Med. J. 2015, 17, e16640. [Google Scholar] [CrossRef] [PubMed]
- Terrone, G.; Bianciardi, E.; Fontana, A.; Pinci, C.; Castellani, G.; Sferra, I.; Forastiere, A.; Merlo, M.; Marinucci, E.; Rinaldi, F.; et al. Psychological Characteristics of Women with Perinatal Depression Who Require Psychiatric Support during Pregnancy or Postpartum: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 5508. [Google Scholar] [CrossRef] [PubMed]
- Le Strat, Y.; Dubertret, C.; Le Foll, B. Prevalence and correlates of major depressive episode in pregnant and postpartum women in the United States. J. Affect. Disord. 2011, 135, 128–138. [Google Scholar] [CrossRef]
- Glasheen, C.; Colpe, L.; Hoffman, V.; Warren, L.K. Prevalence of serious psychological distress and mental health treatment in a national sample of pregnant and postpartum women. Matern. Child Health J. 2015, 19, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Babu, G.R.; Murthy, G.V.S.; Singh, N.; Nath, A.; Rathnaiah, M.; Saldanha, N.; Deepa, R.; Kinra, S. Sociodemographic and Medical Risk Factors Associated with Antepartum Depression. Front. Public Health 2018, 6, 127. [Google Scholar] [CrossRef]
- Helbig, A.; Kaasen, A.; Malt, U.F.; Haugen, G. Does antenatal maternal psychological distress affect placental circulation in the third trimester? PLoS ONE 2013, 8, e57071. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef]
- Batic-Mujanovic, O.; Poric, S.; Pranjic, N.; Ramic, E.; Alibasic, E.; Karic, E. Influence of Unemployment on Mental Health of the Working Age Population. Mater. Socio Medica 2017, 29, 92–96. [Google Scholar] [CrossRef]
- Biaggi, A.; Conroy, S.; Pawlby, S.; Pariante, C.M. Identifying the women at risk of antenatal anxiety and depression: A systematic review. J. Affect. Disord. 2016, 191, 62–77. [Google Scholar] [CrossRef]
- Baranowska-Rataj, A.; Strandh, M. When things go wrong with you, it hurts me too: The effects of partner’s employment status on health in comparative perspective. J. Eur. Soc. Policy 2021, 31, 143–160. [Google Scholar] [CrossRef]
- Goyal, D.; Gay, C.; Lee, K.A. How much does low socioeconomic status increase the risk of prenatal and postpartum depressive symptoms in first-time mothers? Women’s Health Issues 2010, 20, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Hanke, W.; Saurel-Cubizolles, M.J.; Sobala, W.; Kalinka, J. Employment status of pregnant women in central Poland and the risk of preterm delivery and small-for-gestational-age infants. Eur. J. Public Health 2001, 11, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, F.; Arbabisarjou, A.; Boryri, T.; Safarzadeh, A.; Pourkahkhaei, M. The Relationship between Maternal Employment Status and Pregnancy Outcomes. Glob. J. Health Sci. 2016, 8, 53533. [Google Scholar] [CrossRef]
- Cattane, N.; Räikkönen, K.; Anniverno, R.; Mencacci, C.; Riva, M.A.; Pariante, C.M.; Cattaneo, A. Depression, obesity and their comorbidity during pregnancy: Effects on the offspring’s mental and physical health. Mol. Psychiatry 2021, 26, 462–481. [Google Scholar] [CrossRef]
- Ertel, K.A.; Silveira, M.L.; Pekow, P.S.; Dole, N.; Markenson, G.; Chasan-Taber, L. Prepregnancy body mass index, gestational weight gain, and elevated depressive symptoms in a Hispanic cohort. Health Psychol. 2015, 34, 274–278. [Google Scholar] [CrossRef]
- Goldstein, J.M.; Holsen, L.; Huang, G.; Hammond, B.D.; James-Todd, T.; Cherkerzian, S.; Hale, T.M.; Handa, R.J. Prenatal stress-immune programming of sex differences in comorbidity of depression and obesity/metabolic syndrome. Dialogues Clin. Neurosci. 2016, 18, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Molyneaux, E.; Poston, L.; Khondoker, M.; Howard, L.M. Obesity, antenatal depression, diet and gestational weight gain in a population cohort study. Arch. Womens Ment. Health 2016, 19, 899–907. [Google Scholar] [CrossRef]
- Farias, D.R.; Carrilho, T.R.B.; Freitas-Costa, N.C.; Batalha, M.A.; Gonzalez, M.; Kac, G. Maternal mental health and gestational weight gain in a Brazilian Cohort. Sci. Rep. 2021, 11, 10787. [Google Scholar] [CrossRef] [PubMed]
- van Lee, L.; Chia, A.; Phua, D.; Colega, M.; Padmapriya, N.; Bernard, J.Y.; Cai, S.; Tham, E.K.H.; Teoh, O.H.; Goh, D.; et al. Multiple modifiable lifestyle factors and the risk of perinatal depression during pregnancy: Findings from the GUSTO cohort. Compr. Psychiatry 2020, 103, 152210. [Google Scholar] [CrossRef] [PubMed]
- Kołomańska, D.; Zarawski, M.; Mazur-Bialy, A. Physical Activity and Depressive Disorders in Pregnant Women—A Systematic Review. Medicina 2019, 55, 212. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, W.; Zhu, X.; Hu, Z.; Li, S.; Zheng, B.; Xu, H.; Long, W.; Xiong, X. Association between dietary intake and symptoms of depression and anxiety in pregnant women: Evidence from a community-based observational study. Food Sci. Nutr. 2023, 11, 7555–7564. [Google Scholar] [CrossRef]
- Huang, P.; Wei, D.; Xiao, W.; Yuan, M.; Chen, N.; Wei, X.; Xie, J.; Lu, J.; Xia, X.; Lu, M.; et al. Maternal dietary patterns and depressive symptoms during pregnancy: The Born in Guangzhou Cohort Study. Clin. Nutr. 2021, 40, 3485–3494. [Google Scholar] [CrossRef]
- Wu, S.X.; Li, J.; Zhou, D.D.; Xiong, R.G.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Li, H.B. Possible Effects and Mechanisms of Dietary Natural Products and Nutrients on Depression and Anxiety: A Narrative Review. Antioxidants 2022, 11, 2132. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, M.; Colpo, M.; Vermeulen, E.; Elstgeest, L.E.M.; Cabout, M.; Gibson-Smith, D.; Knuppel, A.; Sini, G.; Schoenaker, D.A.J.M.; Mishra, G.D.; et al. Association of a priori dietary patterns with depressive symptoms: A harmonised meta-analysis of observational studies. Psychol. Med. 2020, 50, 1872–1883. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.N.; Liu, Q.; Feng, Y.B.; Li, S.; Wei, X.L.; Atanasov, A.G.; Corke, H.; et al. Health Functions and Related Molecular Mechanisms of Tea Components: An Update Review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, A.; Ziemichód, W.; Herbet, M.; Piątkowska-Chmiel, I. The Role of Diet as a Modulator of the Inflammatory Process in the Neurological Diseases. Nutrients 2023, 15, 1436. [Google Scholar] [CrossRef]
- Awuchi, C.G. Plants, phytochemicals, and natural practices in complementary and alternative system of medicine for treatment of central nervous system disorders. Int. J. Food Prop. 2023, 26, 1190–1213. [Google Scholar] [CrossRef]
- Zhou, D.D.; Luo, M.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Gan, R.Y.; Li, H.B. Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxidative Med. Cell Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Wang, N.; Han, M.; Ban, M.; Sun, T.; Xu, J. Reviewing the role of gut microbiota in the pathogenesis of depression and exploring new therapeutic options. Front. Neurosci. 2022, 16, 1029495. [Google Scholar] [CrossRef] [PubMed]
- Carlessi, A.S.; Borba, L.A.; Zugno, A.I.; Quevedo, J.; Réus, G.Z. Gut microbiota-brain axis in depression: The role of neuroinflammation. Eur. J. Neurosci. 2021, 53, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Manosso, L.M.; Lin, J.; Carlessi, A.S.; Recco, K.C.C.; Quevedo, J.; Gonçalves, C.L.; Réus, G.Z. Sex-related patterns of the gut-microbiota-brain axis in the neuropsychiatric conditions. Brain Res. Bull. 2021, 171, 196–208. [Google Scholar] [CrossRef]
- Bschor, T.; Ising, M.; Erbe, S.; Winkelmann, P.; Ritter, D.; Uhr, M.; Lewitzka, U. Impact of citalopram on the HPA system. A study of the combined DEX/CRH test in 30 unipolar depressed patients. J. Psychiatr. Res. 2012, 46, 111–117. [Google Scholar] [CrossRef]
- Ceruso, A.; Martínez-Cengotitabengoa, M.; Peters-Corbett, A.; Diaz-Gutierrez, M.J.; Martínez-Cengotitabengoa, M. Alterations of the HPA Axis Observed in Patients with Major Depressive Disorder and Their Relation to Early Life Stress: A Systematic Review. Neuropsychobiology 2020, 79, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Hinkelmann, K.; Moritz, S.; Botzenhardt, J.; Muhtz, C.; Wiedemann, K.; Kellner, M.; Otte, C. Changes in cortisol secretion during antidepressive treatment and cognitive improvement in patients with major depression: A longitudinal study. Psychoneuroendocrinology 2012, 37, 685–692. [Google Scholar] [CrossRef]
- Lin, J.; Liu, W.; Guan, J.; Cui, J.; Shi, R.; Wang, L.; Chen, D.; Liu, Y. Latest updates on the serotonergic system in depression and anxiety. Front. Synaptic Neurosci. 2023, 15, 1124112. [Google Scholar] [CrossRef] [PubMed]
- Grote, N.K.; Bridge, J.A.; Gavin, A.R.; Melville, J.L.; Iyengar, S.; Katon, W.J. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch. Gen. Psychiatry 2010, 67, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, U.; Papabathini, S.S.; Kawuki, J.; Obore, N.; Musa, T.H. Depression during pregnancy and the risk of low birth weight, preterm birth and intrauterine growth restriction—An updated meta-analysis. Early Hum. Dev. 2021, 152, 105243. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, J.; Ogbo, F.A.; Hendry, A.; Noble, J.; Page, A.; Early Years Research Group (EYRG). The Impact of Antenatal Depression on Perinatal Outcomes in Australian Women. PLoS ONE 2017, 12, e0169907. [Google Scholar] [CrossRef] [PubMed]
- Neggers, Y.; Goldenberg, R.; Cliver, S.; Hauth, J. The relationship between psychosocial profile, health practices, and pregnancy outcomes. Acta Obstet. Gynecol. Scand. 2006, 85, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Beyene, G.M.; Azale, T.; Gelaye, K.A.; Ayele, T.A. The effect of antenatal depression on birth weight among newborns in South Gondar zone, Northwest Ethiopia: A population-based prospective cohort study. Arch. Public Health 2021, 79, 121. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The World Health Report: 2006: Working Together for Health; World Health Organization: Geneva, Switzerland, 2006; Available online: https://apps.who.int/iris/handle/10665/43432 (accessed on 12 April 2023).
- James, W.P. WHO recognition of the global obesity epidemic. Int. J. Obes. 2008, 32, S120–S126. [Google Scholar] [CrossRef]
- Wang, Y.-P. Clarice Gorenstein. Psychometric properties of the Beck Depression Inventory-II: A comprehensive review. Rev. Bras. Psiquiatr. 2013, 35, 416–431. [Google Scholar] [CrossRef]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal De-pression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Cox, J. Thirty years with the Edinburgh Postnatal Depression Scale: Voices from the past and recommendations for the future. Br. J. Psychiatry 2019, 214, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and bio-logical markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, F.; Panagiotakos, D.B. Healthy indexes in public health practice and research: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, L.A.; Redman, L.M. Weight gain in pregnancy and application of the 2009 IOM guidelines: Toward a uniform ap-proach. Obesity 2015, 23, 507–511. [Google Scholar] [CrossRef]
- Gage, T.B. Classification of births by birth weight and gestational age: An application of multivariate mixture models. Ann. Hum. Biol. 2003, 30, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Pavlidou, E.; Papadopoulou, S.K.; Alexatou, O.; Tsourouflis, G.; Antasouras, G.; Louka, A.; Chatziprodromidou, I.P.; Mentzelou, M.; Sampani, A.; Chrysafi, M.; et al. Association of Gestational Hypertension with Sociodemographic and Anthropometric Factors, Perinatal Outcomes, Breastfeeding Practices, and Mediterranean Diet Adherence: A Cross-Sectional Study. Medicina 2023, 59, 2103. [Google Scholar] [CrossRef] [PubMed]
- Mantzorou, M.; Papandreou, D.; Vasios, G.K.; Pavlidou, E.; Antasouras, G.; Psara, E.; Taha, Z.; Poulios, E.; Giaginis, C. Exclusive Breastfeeding for at Least Four Months Is Associated with a Lower Prevalence of Overweight and Obesity in Mothers and Their Children after 2-5 Years from Delivery. Nutrients 2022, 14, 3599. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.J.; Hartman, T.J.; Adgent, M.; Gardner, K.; Gebretsadik, T.; Moore, P.E.; Davis, R.L.; LeWinn, K.Z.; Bush, N.R.; Tylavsky, F.; et al. Prenatal polyunsaturated fatty acids and child asthma: Effect modification by maternal asthma and child sex. J. Allergy Clin. Immunol. 2020, 145, 800–807.e4. [Google Scholar] [CrossRef]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W.; et al. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef]
- Anastasopoulou, S.V.; Bonotis, K.S.; Hatzoglou, C.; Dafopoulos, K.C.; Gourgoulianis, K.I. Smoking Patterns and Anxiety Factors Among Women Expressing Perinatal Depression. Women’s Health Rep. 2022, 3, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Nisarga, V.; Anupama, M.; Madhu, K.N. Social and obstetric risk factors of antenatal depression: A cross-sectional study from South-India. Asian J. Psychiatry 2022, 72, 103063. [Google Scholar] [CrossRef] [PubMed]
- Roy, U.; Swain, D. A prospective cohort study to assess the prevalence and risk factors of antepartum depression and its effect on maternal and fetal outcome. Asian J. Psychiatry 2024, 91, 103873. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Xiong, R.; Wen, Y.; Liu, L.; Peng, Y.; Xiao, C.; Yin, C.; Liu, W.; Tao, Y.; Jiang, F.; et al. Antenatal depression is associated with perceived stress, family relations, educational and professional status among women in South of China: A multicenter cross-sectional survey. Front. Psychiatry 2023, 14, 1191152. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.A.; Hassan, R.; Shafiq, M.; Sheikh, S. Sociodemographic factors: A major predictor of anxiety and depression among pregnant women. Delhi Psychiatry J. 2015, 18, 86–94. [Google Scholar]
- Smith, M.L.; Kakuhikire, B.; Baguma, C.; Rasmussen, J.D.; Perkins, J.M.; Cooper-Vince, C.; Venkataramani, A.S.; Ashaba, S.; Bangsberg, D.R.; Tsai, A.C. Relative wealth, subjective social status, and their associations with depression: Cross-sectional, population-based study in rural Uganda. SSM Popul. Health 2019, 8, 100448. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.J.; Leung, C. Maternal depressive symptoms, poverty, and young motherhood increase the odds of early depressive and anxiety disorders for children born prematurely. Infant. Ment. Health J. 2021, 42, 586–602. [Google Scholar] [CrossRef] [PubMed]
- Hein, A.; Rauh, C.; Engel, A.; Häberle, L.; Dammer, U.; Voigt, F.; Fasching, P.A.; Faschingbauer, F.; Burger, P.; Beckmann, M.W.; et al. Socioeconomic status and depression during and after pregnancy in the Franconian Maternal Health Evaluation Studies (FRAMES). Arch. Gynecol. Obstet. 2014, 289, 755–763. [Google Scholar] [CrossRef]
- Kurtuluş, Ş.; Can, R.; Sak, Z.H.A. Assessment of the Relationship Between Smoking and Depression in Pregnant Women. J. Immigr. Minor. Health 2021, 23, 536–546. [Google Scholar] [CrossRef]
- Smedberg, J.; Lupattelli, A.; Mårdby, A.C.; Øverland, S.; Nordeng, H. The relationship between maternal depression and smoking cessation during pregnancy—A cross-sectional study of pregnant women from 15 European countries. Arch. Womens Ment. Health 2015, 18, 73–84. [Google Scholar] [CrossRef]
- Leung, B.M.; Kaplan, B.J. Perinatal depression: Prevalence, risks, and the nutrition link—A review of the literature. J. Am. Diet. Assoc. 2009, 109, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Fish and fat intake and prevalence of depressive symptoms during pregnancy in Japan: Baseline data from the Kyushu Okinawa Maternal and Child Health Study. J. Psychiatr. Res. 2013, 47, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients 2023, 15, 2150. [Google Scholar] [CrossRef] [PubMed]
- Antasouras, G.; Papadopoulou, S.K.; Alexatou, O.; Papandreou, D.; Mentzelou, M.; Migdanis, A.; Psara, E.; Migdanis, I.; Chrysafi, M.; Tyrovolas, S.; et al. Adherence to the Mediterranean Diet during Pregnancy: Associations with Sociodemographic and Anthropometric Parameters, Perinatal Outcomes, and Breastfeeding Practices. Medicina 2023, 59, 1547. [Google Scholar] [CrossRef] [PubMed]
- Bizzozero-Peroni, B.; Martínez-Vizcaíno, V.; Fernández-Rodríguez, R.; Jiménez-López, E.; Núñez de Arenas-Arroyo, S.; Saz-Lara, A.; Díaz-Goñi, V.; Mesas, A.E. The impact of the Mediterranean diet on alleviating depressive symptoms in adults: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2024; nuad176ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, V.; Ruohomäki, A.; Hantunen, S.; Keski-Nisula, L.; Luojus, M.K.; Pekkanen, J.; Tuomainen, T.P.; Heinonen, S.; Pasanen, M.; Lehto, S.M. Longitudinal Analyses of Diet Quality and Maternal Depressive Symptoms During Pregnancy: The Kuopio Birth Cohort Study. J. Acad. Nutr. Diet. 2023, 123, 77–86.e4. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.F.O.; Cobucci, R.N.; Gonçalves, A.K.; Lima, S.C.V.C. Systematic review of the association between dietary patterns and perinatal anxiety and depression. BMC Pregnancy Childbirth 2019, 19, 212. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, V.; Giliberti, L.; Giliberti, E.; Grassi, A.; Perin, V.; Parotto, M.; Soldera, G.; Straface, G. The role of gestational weight gain disorders in symptoms of maternal postpartum depression. Int. J. Gynaecol. Obstet. 2021, 153, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Jani, R.; Knight-Agarwal, C.R.; Bloom, M.; Takito, M.Y. The association between pre-pregnancy body mass index, perinatal depression and maternal vitamin D status: Findings from an Australian cohort study. Int. J. Women’s Health 2020, 12, 213–219. [Google Scholar] [CrossRef]
- Dachew, B.A.; Ayano, G.; Betts, K.; Alati, R. The impact of pre-pregnancy BMI on maternal depressive and anxiety symptoms during pregnancy and the postpartum period: A systematic review and meta-analysis. J. Affect. Disord. 2021, 281, 321–330. [Google Scholar] [CrossRef]
- Bazzazian, S.; Riazi, H.; Vafa, M.; Mahmoodi, Z.; Nasiri, M.; Mokhtaryan-Gilani, T.; Ozgoli, G. The relationship between depression, stress, anxiety, and postpartum weight retention: A systematic review. J. Educ. Health Promot. 2021, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Ventriglio, A.; Severo, M.; Petito, A.; Nappi, L.; Iuso, S.; Altamura, M.; Sannicandro, V.; Milano, E.; Arcidiacono, G.; Di Salvatore, M.; et al. The impact of body mass index on the pregnancy outcomes and risk of perinatal depression: Findings from a multicenter Italian study. Eur. Psychiatry 2023, 66, e52. [Google Scholar] [CrossRef] [PubMed]
- Minschart, C.; De Weerdt, K.; Elegeert, A.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; et al. Antenatal Depression and Risk of Gestational Diabetes, Adverse Pregnancy Outcomes, and Postpartum Quality of Life. J. Clin. Endocrinol. Metab. 2021, 106, e3110–e3124. [Google Scholar] [CrossRef]
- Miller, N.E.; Curry, E.; Laabs, S.B.; Manhas, M.; Angstman, K. Impact of gestational diabetes diagnosis on concurrent depression in pregnancy. J. Psychosom. Obstet. Gynaecol. 2021, 42, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Morales-Suárez-Varela, M. The Bidirectional Relationship between Gestational Diabetes and Depression in Pregnant Women: A Systematic Search and Review. Healthcare 2023, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Alder, J.; Fink, N.; Bitzer, J.; Hösli, I.; Holzgreve, W. Depression and anxiety during pregnancy: A risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J. Matern. Fetal Neonatal Med. 2007, 20, 189–209. [Google Scholar] [CrossRef]
- Khanam, R.; Applegate, J.; Nisar, I.; Dutta, A.; Rahman, S.; Nizar, A.; Ali, S.M.; Chowdhury, N.H.; Begum, F.; Dhingra, U.; et al. Burden and risk factors for antenatal depression and its effect on preterm birth in South Asia: A population-based cohort study. PLoS ONE 2022, 17, e0263091. [Google Scholar] [CrossRef] [PubMed]
- Black, M.; Bhattacharya, S.; Philip, S.; Norman, J.E.; McLernon, D.J. Planned Cesarean Delivery at Term and Adverse Outcomes in Childhood Health. JAMA 2015, 314, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Khouj, M.A.; Albasri, S.; Albishri, A.A.; Softa, S.M.; Almaslamani, A.S.; Ahmad, H.M. Prevalence of Stress, Anxiety, and Depression Among Pregnant Women in Jeddah. Cureus 2022, 14, e27174. [Google Scholar] [CrossRef]
- Al Rawahi, A.; Al Kiyumi, M.H.; Al Kimyani, R.; Al-Lawati, I.; Murthi, S.; Davidson, R.; Al Maniri, A.; Al Azri, M. The Effect of Antepartum Depression on the Outcomes of Pregnancy and Development of Postpartum Depression: A prospective cohort study of Omani women. Sultan Qaboos Univ. Med. J. 2020, 20, e179–e186. [Google Scholar] [CrossRef]
- Gow, M.L.; Lam, Y.W.I.; Jebeile, H.; Craig, M.E.; Susic, D.; Henry, A. Antenatal diet quality and perinatal depression: The Microbiome Understanding in Maternity Study (MUMS) cohort. J. Hum. Nutr. Diet. 2023, 36, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Yazici, E.; Kirkan, T.S.; Aslan, P.A.; Aydin, N.; Yazici, A.B. Untreated depression in the first trimester of pregnancy leads to postpartum depression: High rates from a natural follow-up study. Neuropsychiatr. Dis. Treat. 2015, 11, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Farías-Antúnez, S.; Santos, I.S.; Matijasevich, A.; de Barros, A.J.D. Maternal mood symptoms in pregnancy and postpartum depression: Association with exclusive breastfeeding in a population-based birth cohort. Soc. Psychiatry Psychiatr. Epidemiol. 2020, 55, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, M.; Ahn, S. The Impact of Antepartum Depression and Postpartum Depression on Exclusive Breastfeeding: A Systematic Review and Meta-Analysis. Clin. Nurs. Res. 2022, 31, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Glick, I.; Kadish, E.; Rottenstreich, M. Management of Pregnancy in Women of Advanced Maternal Age: Improving Outcomes for Mother and Baby. Int. J. Women’s Health 2021, 13, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.L.; Areia, A.L.; Mota Pinto, A.; Donato, H. Advanced Maternal Age: Adverse Outcomes of Pregnancy, A Meta-Analysis. Acta Med. Port. 2019, 32, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Pavlidou, E.; Papadopoulou, S.K.; Antasouras, G.; Vorvolakos, T.; Alexatou, O.; Tsourouflis, G.; Angelakou, E.P.; Serdari, A.; Grammatikopoulou, M.G.; Psara, E.; et al. Association of COVID-19 Infection with Sociodemographic, Anthropometric and Lifestyle Factors: A Cross-Sectional Study in an Older Adults’ Population Aged over 65 Years Old. Diseases 2023, 11, 165. [Google Scholar] [CrossRef]
- Pavlidou, E.; Papadopoulou, S.K.; Mentzelou, M.; Dakanalis, A.; Vorvolakos, T.; Antasouras, G.; Spanoudaki, M.; Pandi, A.L.; Serdari, A.; Chrysafi, M.; et al. Association of Mediterranean Diet Adherence with Sociodemographic, Anthropometric, and Lifestyle Factors during the COVID-19 Pandemic: A Cross-Sectional Study in Greece. Nutrients 2023, 15, 4123. [Google Scholar] [CrossRef]

| Characteristics (n = 5314) | Pregnant Women’s Perinatal Depression | ||
|---|---|---|---|
| No 3449 (64.9%) | Yes 1865 (35.1%) | p-Value | |
| Pregnant women’s age (mean ± SD; years) | 34.8 ± 5.8 | 34.6 ± 5.4 | p = 0.2364 |
| Maternal nationality (n, %) | p = 0.2973 | ||
| Greek | 3295 (95.5%) | 1793 (96.1%) | |
| Other | 154 (4.5%) | 72 (3.9%) | |
| Maternal education level (n, %) | p = 0.0077 | ||
| Primary education | 1042 (30.2%) | 633 (33.9%) | |
| Secondary education | 1433 (41.6%) | 763 (40.9%) | |
| University studies | 974 (28.2%) | 469 (25.2%) | |
| Family economic status (n, %) | p = 0.0007 | ||
| Low | 1434 (41.6%) | 791 (42.4%) | |
| Medium | 1314 (38.1%) | 773 (51.4%) | |
| High | 701 (20.3%) | 302 (16.2%) | |
| Pregnant women’s marital status (n, %) | p = 0.2427 | ||
| Married | 2410 (69.9%) | 1335 (71.6%) | |
| Other | 1039 (30.1%) | 529 (28.4%) | |
| Pregnant women’s employment status (n, %) | p = 0.5154 | ||
| Employed | 2452 (71.1%) | 1310 (70.2%) | |
| Unemployed | 997 (28.9%) | 555 (29.8%) | |
| Pregnant women’s type of residence | p = 0.0053 | ||
| Urban | 2288 (66.3%) | 1166 (62.5%) | |
| Rural | 1161 (33.7%) | 699 (37.5%) | |
| Pre-pregnancy smoking habits (n, %) | p = 0.0381 | ||
| Not smokers | 2601 (75.4%) | 1358 (72.8%) | |
| Regular smokers | 848 (24.6%) | 507 (27.2%) | |
| Pregnant women’s Mediterranean Diet adherence (n, %) | p < 0.0001 | ||
| Very low | 739 (21.4%) | 591 (31.7%) | |
| Low | 770 (22.3%) | 548 (29.4%) | |
| Moderate | 940 (27.3%) | 388 (20.8%) | |
| High | 1000 (29.0%) | 338 (18.1%) | |
| Parity (n, %) | p = 0.2189 | ||
| Nulliparity | 2394 (69.4%) | 1264 (67.8%) | |
| Multiparity | 1055 (30.6%) | 601 (32.2%) | |
| Maternal pre-pregnancy BMI status (n, %) | p < 0.0001 | ||
| Normal weight | 2612 (75.7%) | 1234 (66.2%) | |
| Overweight | 553 (16.0%) | 391 (21.0%) | |
| Obese | 283 (8.3%) | 239 (12.8%) | |
| Gestational weight gain (n, %) | p < 0.0001 | ||
| Low | 193 (5.6%) | 92 (4.9%) | |
| Normal | 2373 (68.8%) | 1157 (62.1%) | |
| Excessive | 883 (25.6%) | 616 (33.0%) | |
| Preterm birth (<37th week; n, %) | p = 0.0008 | ||
| No | 2867 (83.1%) | 1481 (79.4%) | |
| Yes | 582 (16.9%) | 384 (20.6%) | |
| Childbirth weight status (n, %) | p < 0.0001 | ||
| <2500 g | 236 (6.8%) | 143 (7.7%) | |
| 2500–4000 g | 2752 (79.8%) | 1355 (76.6%) | |
| >4000 g | 461 (13.4%) | 367 (19.7%) | |
| Gestational diabetes (n, %) | p = 0.0008 | ||
| No | 3242 (94.0%) | 1699 (91.1%) | |
| Yes | 207 (6.0%) | 166 (8.9%) | |
| Gestational hypertension (n, %) | p = 0.8397 | ||
| No | 3140 (91.0%) | 1701 (91.2%) | |
| Yes | 309 (9.0%) | 164 (8.8%) | |
| Kind of delivery (n, %) | p = 0.0001 | ||
| Vaginal | 1565 (45.4%) | 736 (39.5%) | |
| Caesarean section | 1884 (54.6%) | 1129 (60.5%) | |
| Exclusive breastfeeding (n, %) | p < 0.0001 | ||
| No | 1347 (39.1%) | 1271 (68.1%) | |
| Yes | 2102 (60.9%) | 594 (31.9%) | |
| Postpartum depression (n, %) | p < 0.0001 | ||
| No | 3152 (91.4%) | 1636 (87.7%) | |
| Yes | 297 (8.6%) | 229 (12.3%) | |
| Childhood asthma (n, %) | p < 0.0001 | ||
| No | 3316 (96.1%) | 1732 (92.9%) | |
| Yes | 133 (3.9%) | 133 (7.1%) | |
| Childhood diabetes mellitus type 1 (n, %) | p = 0.1850 | ||
| No | 3289 (95.4%) | 1793 (96.1%) | |
| Yes | 160 (4.6%) | 72 (3.9%) | |
| Characteristics, (n = 5314) | Pregnant Women’s Perinatal Depression (No vs. Yes) | |
|---|---|---|
| HR * (95% CI **) | p-Value | |
| Pregnant women’s age (over/below mean) | 1.02 (0.39–1.72) | p = 0.5833 |
| Maternal nationality (Greek/Other) | 0.97 (0.25–1.68) | p = 0.7632 |
| Maternal education level (University/Primary and Secondary) | 1.28 (0.87–1.71) | p = 0.0276 |
| Family economic status (Low and Medium/High) | 1.36 (1.03–1.68) | p = 0.0120 |
| Pregnant women’s marital status (Married/Other) | 0.96 (0.25–1.69) | p = 0.6874 |
| Pregnant women’s employment status (Employed/Unemployed) | 0.95 (0.21–1.75) | p = 0.7097 |
| Pregnant women’s type of residence (Rural/Urban) | 1.31 (0.98–1.67) | p = 0.0783 |
| Pre-pregnancy smoking habits (Not Smokers/Regular Smokers) | 1.12 (0.78–1.57) | p = 0.1843 |
| Pregnant women’s Mediterranean Diet adherence (Moderate + High/Very Low + Low) | 2.18 (1.97–2.40) | p = 0.0011 |
| Parity (Nulliparity/Multiparity) | 0.97 (0.28–1.73) | p = 0.4509 |
| Maternal pre-pregnancy BMI status (Normal/Overweight + Obese) | 2.05 (1.87–2.24) | p = 0.0005 |
| Gestational weight gain (Low + Normal/Excessive) | 1.86 (1.65–2.07) | p = 0.0003 |
| Premature childbirth (No/Yes) | 1.48 (1.21–1.74) | p = 0.0014 |
| Childbirth weight status (<4000 g/>4000 g) | 1.77 (1.56–1.98) | p = 0.0017 |
| Gestational diabetes (No/Yes) | 1.55 (1.29–1.82) | p = 0.0026 |
| Gestational hypertension (No/Yes) | 0.96 (0.22–1.87) | p = 0.9372 |
| Type of delivery (Vaginal/Caesarean section) | 1.63 (1.32–1.95) | p = 0.0025 |
| Exclusive breastfeeding (No/Yes) | 1.95 (1.73–2.18) | p = 0.0034 |
| Postpartum depression (No/Yes) | 2.48 (2.31–2.62) | p = 0.0002 |
| Childhood asthma (No/Yes) | 1.72 (1.49–1.98) | p = 0.0021 |
| Childhood diabetes mellitus type 1 (No/Yes) | 0.97 (0.36–1.59) | p = 0.3875 |
| Characteristics, (n = 5314) | Pregnant Women’s Perinatal Depression (No vs. Yes) | |
|---|---|---|
| HR * (95% CI **) | p-Value | |
| Maternal education level (University/Primary and Secondary) | 1.17 (0.72–1.94) | p = 0.0835 |
| Family economic status (Low and Medium/High) | 1.22 (0.81–1.92) | p = 0.1453 |
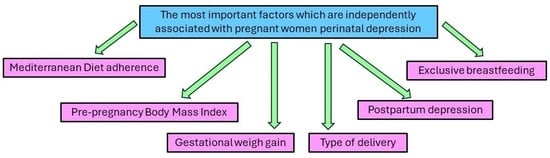
| Pregnant women’s Mediterranean Diet adherence (Moderate + High/Very Low + Low) | 2.24 (1.99–2.43) | p = 0.0018 |
| Maternal pre-pregnancy BMI status (Normal/Overweight + Obese) | 2.09 (1.82–2.31) | p = 0.0013 |
| Gestational weight gain (Low + Normal/Excessive) | 1.88 (1.63–2.11) | p = 0.0008 |
| Premature childbirth (No/Yes) | 1.43 (1.01–1.98) | p = 0.1022 |
| Childbirth weight status (<4000 g/>4000 g) | 1.75 (1.26–2.23) | p = 0.0746 |
| Gestational diabetes (No/Yes) | 1.51 (1.12–1.98) | p = 0.1108 |
| Type of delivery (Vaginal/Caesarean section) | 1.65 (1.28–2.05) | p = 0.0291 |
| Exclusive breastfeeding (No/Yes) | 1.98 (1.65–2.29) | p = 0.0201 |
| Postpartum depression (No/Yes) | 2.55 (2.21–2.62) | p = 0.0015 |
| Childhood asthma (No/Yes) | 1.61 (1.30–2.17) | p = 0.0976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacovides, C.; Papadopoulou, S.K.; Pavlidou, E.; Dakanalis, A.; Alexatou, O.; Vorvolakos, T.; Lechouritis, E.; Papacosta, E.; Chrysafi, M.; Mitsiou, M.; et al. Association of Pregnant Women’s Perinatal Depression with Sociodemographic, Anthropometric and Lifestyle Factors and Perinatal and Postnatal Outcomes: A Cross-Sectional Study. J. Clin. Med. 2024, 13, 2096. https://doi.org/10.3390/jcm13072096
Jacovides C, Papadopoulou SK, Pavlidou E, Dakanalis A, Alexatou O, Vorvolakos T, Lechouritis E, Papacosta E, Chrysafi M, Mitsiou M, et al. Association of Pregnant Women’s Perinatal Depression with Sociodemographic, Anthropometric and Lifestyle Factors and Perinatal and Postnatal Outcomes: A Cross-Sectional Study. Journal of Clinical Medicine. 2024; 13(7):2096. https://doi.org/10.3390/jcm13072096
Chicago/Turabian StyleJacovides, Constantina, Sousana K. Papadopoulou, Eleni Pavlidou, Antonios Dakanalis, Olga Alexatou, Theofanis Vorvolakos, Eleftherios Lechouritis, Elena Papacosta, Maria Chrysafi, Maria Mitsiou, and et al. 2024. "Association of Pregnant Women’s Perinatal Depression with Sociodemographic, Anthropometric and Lifestyle Factors and Perinatal and Postnatal Outcomes: A Cross-Sectional Study" Journal of Clinical Medicine 13, no. 7: 2096. https://doi.org/10.3390/jcm13072096