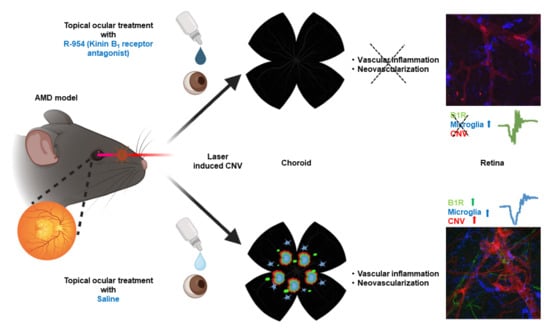
Prevention of Inflammation, Neovascularization, and Retinal Dysfunction by Kinin B1 Receptor Antagonism in a Mouse Model of Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Experimental CNV
2.3. Topical Administration of B1R Antagonist R-954
2.4. Electroretinogram Recordings
2.5. Electroretinogram Analysis
2.6. Flat Mount Dissection and Immunofluorescent Staining
2.7. Quantitative Measurement of Choroidal Neovascularization and Inflammation
2.8. Statistical Analysis
3. Results
3.1. Development of Neovascularization in the Murine Model of AMD
| Choroid | Retina | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline Treated CNV | R-954 Treated CNV | Saline Treated CNV | R-954 Treated CNV | |||||||||
| Isolectin GS-IB4 | ||||||||||||
| 7-day | 65 | ± | 4 | 13 | ± | 1 * | 151 | ± | 7 | 34 | ± | 3 * |
| 14-day | 162 | ± | 13 | 26 | ± | 2 * | 77 | ± | 2 | 17 | ± | 3 * |
| 21-day | 248 | ± | 12 | 30 | ± | 4 * | 157 | ± | 22 | 31 | ± | 5 * |
| B1R | ||||||||||||
| 7-day | 39 | ± | 2 | 13 | ± | 1 * | 204 | ± | 4 | 23 | ± | 1 * |
| 14-day | 95 | ± | 4 | 8 | ± | 1 * | 217 | ± | 15 | 13 | ± | 1 * |
| 21-day | 172 | ± | 10 | 14 | ± | 1 * | 255 | ± | 18 | 24 | ± | 2 * |
| Iba1 | ||||||||||||
| 7-day | 51 | ± | 5 | 15 | ± | 1 * | 133 | ± | 4 | 11 | ± | 2 * |
| 14-day | 82 | ± | 4 | 3 | ± | 1 * | 168 | ± | 20 | 11 | ± | 1 * |
| 21-day | 33 | ± | 16 | 20 | ± | 1 * | 241 | ± | 25 | 9 | ± | 1 * |
3.2. R-954 Inhibits Choroidal Neovascularization
3.3. R-954 Prevents Retinal Vascular Bed Alteration
3.4. Cellular Localization of B1R in CNV Retina
3.5. R-954 Prevents Retinal Dysfunction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pugazhendhi, A.; Hubbell, M.; Jairam, P.; Ambati, B. Neovascular Macular Degeneration: A Review of Etiology, Risk Factors, and Recent Advances in Research and Therapy. Int. J. Mol. Sci. 2021, 22, 1170. [Google Scholar] [CrossRef] [PubMed]
- Tsujinaka, H.; Fu, J.; Shen, J.; Yu, Y.; Hafiz, Z.; Kays, J.; McKenzie, D.; Cardona, D.; Culp, D.; Peterson, W.; et al. Sustained treatment of retinal vascular diseases with self-aggregating sunitinib microparticles. Nat. Commun. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Jhingan, M.; Singh, S.R.; Samanta, A.; Arora, S.; Tucci, D.; Amarasekera, S.; Cagini, C.; Lupidi, M.; Chhablani, J. Drusen ooze: Predictor for progression of dry age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 2687–2694. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, A.; Mahdi, L.; Musat, O. Age-related macular degeneration. Rom. J. Ophthalmol. 2015, 59, 74–77. [Google Scholar]
- Rastoin, O.; Pagès, G.; Dufies, M. Experimental Models in Neovascular Age Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4627. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: The Age-Related Eye Disease Study Report Number 6. Am. J. Ophthalmol. 2001, 132, 668–681. [Google Scholar] [CrossRef]
- Tan, W.; Zou, J.; Yoshida, S.; Jiang, B.; Zhou, Y. The Role of Inflammation in Age-Related Macular Degeneration. Int. J. Biol. Sci. 2020, 16, 2989–3001. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, N.K. Inflammation and retinal degenerative diseases. Neural Regen. Res. 2023, 18, 513–518. [Google Scholar] [CrossRef]
- Othman, R.; Cagnone, G.; Joyal, J.S.; Vaucher, E.; Couture, R. Kinins and Their Receptors as Potential Therapeutic Targets in Retinal Pathologies. Cells 2021, 10, 1913. [Google Scholar] [CrossRef]
- Brusco, I.; Fialho, M.F.P.; Becker, G.; Brum, E.S.; Favarin, A.; Marquezin, L.P.; Serafini, P.T.; Oliveira, S.M. Kinins and their B(1) and B(2) receptors as potential therapeutic targets for pain relief. Life Sci. 2023, 314, 121302. [Google Scholar] [CrossRef]
- Lacoste, B.; Tong, X.K.; Lahjouji, K.; Couture, R.; Hamel, E. Cognitive and cerebrovascular improvements following kinin B1 receptor blockade in Alzheimer’s disease mice. J. Neuroinflamm. 2013, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Girolami, J.P.; Bouby, N.; Richer-Giudicelli, C.; Alhenc-Gelas, F. Kinins and Kinin Receptors in Cardiovascular and Renal Diseases. Pharmaceuticals 2021, 14, 240. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Rousseau, J.; Kwon, D.; Benard, F.; Lin, K.S. A Systematic Review of Molecular Imaging Agents Targeting Bradykinin B1 and B2 Receptors. Pharmaceuticals 2020, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Dagnino, A.P.A.; Campos, M.M.; Silva, R.B.M. Kinins and Their Receptors in Infectious Diseases. Pharmaceuticals 2020, 13, 215. [Google Scholar] [CrossRef]
- Hachana, S.; Bhat, M.; Sénécal, J.; Huppé-Gourgues, F.; Couture, R.; Vaucher, E. Expression, distribution and function of kinin B(1) receptor in the rat diabetic retina. Br. J. Pharmacol. 2018, 175, 968–983. [Google Scholar] [CrossRef]
- Hachana, S.; Fontaine, O.; Sapieha, P.; Lesk, M.; Couture, R.; Vaucher, E. The Effects of Anti-VEGF and Kinin B1 Receptor Blockade on Retinal Inflammation in Laser-Induced Choroidal Neovascularization. Br. J. Pharmacol. 2020, 177, 1949–1966. [Google Scholar] [CrossRef]
- Othman, R.; Berbari, S.; Vaucher, E.; Couture, R. Differential Expression of Kinin Receptors in Human Wet and Dry Age-Related Macular Degeneration Retinae. Pharmaceuticals 2020, 13, 130. [Google Scholar] [CrossRef]
- Sun, J.K.; Maturi, R.K.; Boyer, D.S.; Wells, J.A.; Gonzalez, V.H.; Tansley, R.; Hernandez, H.; Maetzel, A.; Feener, E.P.; Aiello, L.P. One-Time Intravitreal Injection of KVD001, a Plasma Kallikrein Inhibitor, in Patients with Central-Involved Diabetic Macular Edema and Reduced Vision: An Open-Label Phase 1B Study. Ophthalmol. Retin. 2019, 3, 1107–1109. [Google Scholar] [CrossRef]
- Gong, Y.; Li, J.; Sun, Y.; Fu, Z.; Liu, C.H.; Evans, L.; Tian, K.; Saba, N.; Fredrick, T.; Morss, P.; et al. Optimization of an Image-Guided Laser-Induced Choroidal Neovascularization Model in Mice. PLoS ONE 2015, 10, e0132643. [Google Scholar] [CrossRef]
- Xin, X.; Zhu, Y.; Xi, R.; Hao, Y. Establishing a mouse model of choroidal neovascularization to study the therapeutic effect of levotinib and its mechanism. Saudi J. Biol. Sci. 2020, 27, 2491–2497. [Google Scholar] [CrossRef]
- Shah, R.S.; Soetikno, B.T.; Lajko, M.; Fawzi, A.A. A Mouse Model for Laser-induced Choroidal Neovascularization. J. Vis. Exp. 2015, 106, e53502. [Google Scholar] [CrossRef]
- Gobeil, F., Jr.; Sirois, P.; Regoli, D. Preclinical pharmacology, metabolic stability, pharmacokinetics and toxicology of the peptidic kinin B1 receptor antagonist R-954. Peptides 2014, 52, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Cécyre, B.; Bachand, I.; Papineau, F.; Brochu, C.; Casanova, C.; Bouchard, J.F. Cannabinoids affect the mouse visual acuity via the cannabinoid receptor type 2. Sci. Rep. 2020, 10, 15819. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, D.; Yasumura, D.; Benchorin, G.; Matthes, M.T.; Feng, W.; Nguyen, N.M.; Sedano, C.D.; Calton, M.A.; LaVail, M.M. Tyro3 Modulates Mertk-Associated Retinal Degeneration. PLoS Genet. 2015, 11, e1005723. [Google Scholar] [CrossRef]
- Guthrie, M.J.; Osswald, C.R.; Valio, N.L.; Mieler, W.F.; Kang-Mieler, J.J. Objective area measurement technique for choroidal neovascularization from fluorescein angiography. Microvasc. Res. 2014, 91, 1–7. [Google Scholar] [CrossRef]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef]
- Nozaki, M.; Raisler, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z.; et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. USA 2006, 103, 2328–2333. [Google Scholar] [CrossRef]
- Rohrer, B.; Long, Q.; Coughlin, B.; Wilson, R.B.; Huang, Y.; Qiao, F.; Tang, P.H.; Kunchithapautham, K.; Gilkeson, G.S.; Tomlinson, S. A targeted inhibitor of the alternative complement pathway reduces angiogenesis in a mouse model of age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3056–3064. [Google Scholar] [CrossRef]
- Hirano, Y.; Suzuki, N.; Tomiyasu, T.; Kurobe, R.; Yasuda, Y.; Esaki, Y.; Yasukawa, T.; Yoshida, M.; Ogura, Y. Multimodal Imaging of Microvascular Abnormalities in Retinal Vein Occlusion. J. Clin. Med. 2021, 10, 405. [Google Scholar] [CrossRef]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in Retinal Degeneration. Front. Immunol. 2019, 10, 1975. [Google Scholar] [CrossRef]
- Guo, L.; Choi, S.; Bikkannavar, P.; Cordeiro, M.F. Microglia: Key Players in Retinal Ageing and Neurodegeneration. Front. Cell Neurosci. 2022, 16, 804782. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Feng, S.; Qin, H.; Yan, L.; Zheng, C.; Yao, K. Microglia: The breakthrough to treat neovascularization and repair blood-retinal barrier in retinopathy. Front. Mol. Neurosci. 2023, 16, 1100254. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y. The Multiple Roles of Pericytes in Vascular Formation and Microglial Functions in the Brain. Life 2022, 12, 1835. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, A.; Bringmann, A. New functions of Müller cells. Glia 2013, 61, 651–678. [Google Scholar] [CrossRef]
- Fernández-Sánchez, L.; Lax, P.; Campello, L.; Pinilla, I.; Cuenca, N. Astrocytes and Müller Cell Alterations During Retinal Degeneration in a Transgenic Rat Model of Retinitis Pigmentosa. Front. Cell Neurosci. 2015, 9, 484. [Google Scholar] [CrossRef]
- Carpi-Santos, R.; de Melo Reis, R.A.; Gomes, F.C.A.; Calaza, K.C. Contribution of Müller Cells in the Diabetic Retinopathy Development: Focus on Oxidative Stress and Inflammation. Antioxidants 2022, 11, 617. [Google Scholar] [CrossRef]
- Tisi, A.; Feligioni, M.; Passacantando, M.; Ciancaglini, M.; Maccarone, R. The Impact of Oxidative Stress on Blood-Retinal Barrier Physiology in Age-Related Macular Degeneration. Cells 2021, 10, 64. [Google Scholar] [CrossRef]
- Gauthier, M.; Simard, A.B.; Polosa, A.; Dorfman, A.L.; Qian, C.X.; Lina, J.M.; Lachapelle, P. Resting state electroretinography: An innovative approach to intrinsic retinal function monitoring. Front. Physiol. 2022, 13, 931147. [Google Scholar] [CrossRef]
- Zahra, S.; Murphy, M.J.; Crewther, S.G.; Riddell, N. Flash Electroretinography as a Measure of Retinal Function in Myopia and Hyperopia: A Systematic Review. Vision 2023, 7, 15. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Gericke, A. Age-Related Macular Degeneration: Role of Oxidative Stress and Blood Vessels. Int. J. Mol. Sci. 2021, 22, 1296. [Google Scholar] [CrossRef]
- Toma, C.; De Cillà, S.; Palumbo, A.; Garhwal, D.P.; Grossini, E. Oxidative and Nitrosative Stress in Age-Related Macular Degeneration: A Review of Their Role in Different Stages of Disease. Antioxidants 2021, 10, 653. [Google Scholar] [CrossRef]
- Mitchell, P. A systematic review of the efficacy and safety outcomes of anti-VEGF agents used for treating neovascular age-related macular degeneration: Comparison of ranibizumab and bevacizumab. Curr. Med. Res. Opin. 2011, 27, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, S.; Clearfield, E.; Soliman, M.K.; Sadiq, M.A.; Baldwin, A.J.; Hanout, M.; Agarwal, A.; Sepah, Y.J.; Do, D.V.; Nguyen, Q.D. Aflibercept for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2016, 2, CD011346. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.; Lanitis, T.; Cele, C.; Toro-Diaz, H.; Gibson, A.; Kuznik, A. Intravitreal Aflibercept Versus Ranibizumab for Wet Age-Related Macular Degeneration: A Cost-effectiveness Analysis. J. Manag. Care Spec. Pharm. 2018, 24, 608–616. [Google Scholar] [CrossRef]
- Fontaine, O.; Olivier, S.; Descovich, D.; Cordahi, G.; Vaucher, E.; Lesk, M.R. The Effect of Intravitreal Injection of Bevacizumab on Retinal Circulation in Patients with Neovascular Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7400–7405. [Google Scholar] [CrossRef]
- Sene, A.; Chin-Yee, D.; Apte, R.S. Seeing through VEGF: Innate and adaptive immunity in pathological angiogenesis in the eye. Trends Mol. Med. 2015, 21, 43–51. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, M.; Shirzad, S.; Fofana, A.-R.K.; Gobeil, F., Jr.; Couture, R.; Vaucher, E. Prevention of Inflammation, Neovascularization, and Retinal Dysfunction by Kinin B1 Receptor Antagonism in a Mouse Model of Age-Related Macular Degeneration. J. Clin. Med. 2023, 12, 6213. https://doi.org/10.3390/jcm12196213
Bhat M, Shirzad S, Fofana A-RK, Gobeil F Jr., Couture R, Vaucher E. Prevention of Inflammation, Neovascularization, and Retinal Dysfunction by Kinin B1 Receptor Antagonism in a Mouse Model of Age-Related Macular Degeneration. Journal of Clinical Medicine. 2023; 12(19):6213. https://doi.org/10.3390/jcm12196213
Chicago/Turabian StyleBhat, Menakshi, Shima Shirzad, Abdel-Rahamane Kader Fofana, Fernand Gobeil, Jr., Réjean Couture, and Elvire Vaucher. 2023. "Prevention of Inflammation, Neovascularization, and Retinal Dysfunction by Kinin B1 Receptor Antagonism in a Mouse Model of Age-Related Macular Degeneration" Journal of Clinical Medicine 12, no. 19: 6213. https://doi.org/10.3390/jcm12196213