Gastrointestinal Transit Times in Health as Determined Using Ingestible Capsule Systems: A Systematic Review
Abstract
:1. Introduction
- a literature review of IC systems used clinically and in research for the assessment of GI motility;
- a systematic review of studies utilising IC systems to measure and report gastric emptying time (GET), small intestinal transit time (SITT), colonic transit time (CTT) and whole-gut transit time (WGTT) in healthy volunteers.
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Study Selection
2.5. Data Collection, Data Items and Summary Measures
2.6. Assessment of Risk of Bias
2.7. Synthesis of Results
3. Results
3.1. Results of the Literature Review
3.2. Study Selection for the Systematic Review
3.3. Characteristics of Included Studies
| Author | Publication Year | Country | Study Design * | ITT HV Sample Size (N) | Female (N) | Actual HV Sample Size (N) | Age (Years) | Min | ICS Sensor Type | Bowel Prep | Capsule Ingested with Meal? | Ingestion Meal kCal | Capsule Ingestion Time | Fasting Duration after Capsule Ingestion (Hours) | GET | SITT | CTT | WGTT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Evans [71] | 1988 | UK | Case series | 72 | 21 | 66 (SITT) 32 (WGTT) | Median: 26 Range: 20–83 | RTC | pH | Overnight fast | No—only water | None | Morning (08:30) | Subjects fasted until capsule left stomach (indicated by pH rise) | ✓ | ✓ | ||
| Fallingborg [72] | 1989 | Denmark | Case series | 39 | 21 | 39 | Median: 33 Range: 18–65 | RTC | pH | Overnight fast | Not specified | Not specified | Morning (~08:00) | Subjects fasted until capsule left stomach as indicated by pH rise | ✓ | |||
| Goldstein [63] † | 2007 | Multicentre (USA, Israel) | Randomised controlled trial | 580 | 66 | 113† | Mean: 32.2 ± 10.0 Range: 18–65 | PillCam SB | Imaging | 12 h fast | Not specified | Not specified | Not specified | Not specified | ✓ | |||
| Malagelada [64] | 2008 | Spain | Observational cohort study | 50 | 27 | 34 | Range: 18–36 | PillCam SB | Imaging | Overnight fast | Not specified | None | Not specified | Liquid meal (300 mL (1 kCal/mL)) ingested 60 min after capsule ingestion. | ✓ | |||
| Hocke [30] | 2009 | Germany | Case series | 21 | 10 | 21 | Female mean 35.8 ± 11.6 Male mean 40.4 ± 13.6 | 3D-MAGMA | Magnetic | Overnight fast | No—only water | None | Morning (between 08:00 and 12:00) | Not specified | ✓ | |||
| Hooks [75] | 2009 | USA | Randomised controlled trial | 40 | 13 | 20 (GET) † 19 (SITT) † | 35.4 | PillCam SB | Imaging | 8 h fast | Not specified | Not specified | Not specified | Not specified | ✓ | ✓ | ||
| Fujimori [78] | 2010 | Japan | Before-after study with no control group | 55 | 0 | 55 | Mean 37 ± 8 | PillCam SB | Imaging | 12 h fast | Not specified | Not specified | Not specified | Not specified | ✓ | ✓ | ||
| Malagelada [65] | 2012 | Spain | Observational cohort study | 70 | 39 | 52 | Range: 18–66 | PillCam SB | Imaging | Overnight fast | Not specified | None | Morning | Liquid meal ingested (300 mL 1 kCal/mL) 45 min after capsule ingestion. | ✓ | ✓ | ||
| van der Schaar [55] | 2013 | Multicentre (The Netherlands, USA) | Case series | 20 | 14 | 20 | Study 1 mean: 21.6 Study 2 mean: 20.6 Range: 19–25 | IntelliCap | pH and temperature | Overnight fast | No—only water | None | Morning | 4 h | ✓ | ✓ | ✓ | ✓ |
| Haase [67] | 2014 | Multicentre (Denmark, Switzerland, UK, Czech Republic) | Case series | 20 | 10 | Capsule 1: 20 Capsule 2: 19 (GET and SITT) 17 (CTT) 17 (WGTT) Capsule 3: 17 (GET) 18 (SITT) 15 (CTT and WGTT) | Median: 32 Range: 26–52 | 3D-Transit | Electromagnetic | Overnight fast | Yes | 354 kCal for female subjects 602 kCal for male subjects. | Capsule 1: Day 1 morning Capsule 2: Day 1 evening Capsule 3: Day 2 morning | 6 h | ✓ | ✓ | ✓ | ✓ |
| Koziolek [54] | 2015 | Germany | Case series | 20 | 11 | 19 | Mean: 26.0 ± 4.1 Range: 21–34 | WMC | pH, temperature and pressure | At least 10 h fast | Yes | 964 kCal | Morning | 100 mL water 1, 2, 3 and 4 h after capsule ingestion. Lunch served 4.5 h after capsule ingestion (1000 kCal) | ✓ | |||
| Malagelada [66] | 2015 | Spain | Observational cohort study | 136 | 75 | 132 | Range: 16–65 | PillCam SB | Imaging | Overnight fast | Not specified | None | Morning | Liquid meal ingested (300 mL 1 kCal/mL) 45 min after capsule ingestion. | ✓ | |||
| Wang [6] | 2015 | Multicentre (UK, Sweden, USA) | Observational cohort study | 215 | 87 | 199 (GET, SITT) 182 (CTT) 194 (WGTT) | Median: 33 Range: 23–49 | WMC | pH, temperature and pressure | Overnight fast | Yes | Between 255 kCal and 262 kCal | Morning | 6 h | ✓ | ✓ | ✓ | ✓ |
| Jianqin [76] ‡ | 2016 | Multicentre (China, Australia, New Zealand) | Randomised controlled trial | 45 | 24 | 40 ‡ | Mean: 46.6 ± 14 | OMOM | Imaging | Not specified | Not specified | Not specified | Not specified | Not specified | ✓ | ✓ | ✓ | |
| Monnard [69] | 2017 | Switzerland | Observational cohort study | 27 | 18 | 21 | Mean: 25 ± 6 | CorTemp | Temperature | Not specified | No—only water | None | Afternoon (between 16:00 and 18:00) | Evening meal consumed 2.5–4 h after capsule ingestion | ✓ | |||
| Sakurai [70] | 2018 | Japan | Observational cohort study | 150 | 74 | 148 | Mean: 48.8 ± 6.5 | PillCam SB | Imaging | Not specified | Not specified | Not specified | Not specified | Not specified | ✓ | ✓ | ||
| Nandhra [5] | 2020 | Multicentre (UK, Denmark, Australia, Switzerland) | Observational cohort study | 111 | 58 | 104 (GET) 111 (SITT, CTT, WGTT) | Median: 40 Range: 21–88 | 3D-Transit | Electromagnetic | Overnight fast | Yes | Between 255 kCal and 602 kCal | Morning | 6 h | ✓ | ✓ | ✓ | ✓ |
| O’Grady [74] | 2020 | Ireland | Case series | 71 | 40 | 71 | Mean: 30.5 ± 6.7 Range: 19–40 | PillCam SB | Imaging | Overnight fast | No—only water | None | Morning | 4 h | ✓ | ✓ | ||
| Mark [68] | 2021 | Multicentre (Denmark, UK) | Randomised controlled trial | 21 | 0 | 17 (GET, SITT, CTT) † 18 (WGTT) † | Median: 25 Range: 20–30 | 3D-Transit | Electromagnetic | Overnight fast | Yes | 285 kCal | Not specified | 6 h | ✓ | ✓ | ✓ | ✓ |
| Sangnes [79] § | 2021 | Norway | Observational case-control study | 26 | 14 | 26 | Mean: 42 ± 15 | WMC | pH, temperature and pressure | Overnight fast | Yes | 260 kCal | Morning | 6 h | ✓ | ✓ | ✓ | ✓ |
| Thwaites [61] | 2022 | Australia and New Zealand | Observational cohort study | Primary cohort: 26 Validation cohort: 24 Tandem gas-sensing capsule cohort: 20 | Primary cohort: 10 Validation cohort: 18 Tandem gas-sensing capsule cohort: 6 | Primary cohort: 21–25 Validation cohort: 14–20 Tandem gas-sensing capsule cohort: 17–18 | Primary cohort: Median: 35 Range: 31–39 Validation cohort: Median: 25 Range: 23–30 Tandem gas-sensing capsule cohort: Median: 35 Range: 29–39 | WMC and Atmo gas sensing capsule | pH, temperature and pressure Gas sensing | Overnight fast | Yes | 1092 kJ (260 kCal) | Morning | 6 h | ✓ | ✓ | ✓ | ✓ |
| Creedon [77] § | 2022 | UK | Randomised controlled trial | Control group ITT: 26 | 25 | Control group ITT: 14 | Control group: Mean: 27.9 ± 5 | WMC | pH, temperature and pressure | Overnight fast | Yes | 255 kCal | Morning | 6 h | ✓ | ✓ | ✓ | ✓ |
3.4. Risk of Bias and Quality of Included Studies
3.5. Synthesis of Results
3.5.1. Gastrointestinal Transit Times: Gastric Emptying Time





3.5.2. Small Intestinal Transit Time
3.5.3. Colonic Transit Time
3.5.4. Whole-Gut Transit Time
4. Discussion
4.1. Summary of Evidence
4.1.1. Gastric Emptying Time
4.1.2. Small Intestinal Transit Time
4.1.3. Colonic Transit Time
4.1.4. Whole-Gut Transit Time
4.1.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mackay, R.S.; Jacobson, B. Endoradiosonde. Nature 1957, 179, 1239–1240. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, B.; Mackay, R.S. A pH-endoradiosonde. Lancet 1957, 272, 1224. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Ha, N.; Ou, J.Z.; Berean, K.J. Ingestible Sensors. ACS Sens. 2017, 2, 468–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronlund, D.; Poulsen, J.L.; Sandberg, T.H.; Olesen, A.E.; Madzak, A.; Krogh, K.; Frokjaer, J.B.; Drewes, A.M. Established and emerging methods for assessment of small and large intestinal motility. Neurogastroenterol. Motil. 2017, 29, e13008. [Google Scholar] [CrossRef]
- Nandhra, G.K.; Mark, E.B.; Di Tanna, G.L.; Haase, A.M.; Poulsen, J.; Christodoulides, S.; Kung, V.; Klinge, M.W.; Knudsen, K.; Borghammer, P.; et al. Normative values for region-specific colonic and gastrointestinal transit times in 111 healthy volunteers using the 3D-Transit electromagnet tracking system: Influence of age, gender, and body mass index. Neurogastroenterol. Motil. 2020, 32, e13734. [Google Scholar] [CrossRef]
- Wang, Y.T.; Mohammed, S.D.; Farmer, A.D.; Wang, D.; Zarate, N.; Hobson, A.R.; Hellstrom, P.M.; Semler, J.R.; Kuo, B.; Rao, S.S.; et al. Regional gastrointestinal transit and pH studied in 215 healthy volunteers using the wireless motility capsule: Influence of age, gender, study country and testing protocol. Aliment. Pharmacol. Ther. 2015, 42, 761–772. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojaverian, P.; Vlasses, P.H.; Parker, S.; Warner, C. Influence of single and multiple doses of oral ranitidine on the gastric transit of an indigestible capsule in humans. Clin. Pharmacol. Ther. 1990, 47, 382–388. [Google Scholar] [CrossRef]
- Heidelberg, M. Heidelberg pH Diagnostic Systems. Available online: https://www.phcapsule.com (accessed on 25 June 2021).
- Andres, M.R., Jr.; Bingham, J.R. Tubeless gastric analysis with a radiotelemetering pill (Heidelberg capsule). Can. Med. Assoc. J. 1970, 102, 1087–1089. [Google Scholar]
- Colson, R.H.; Watson, B.W.; Fairclough, P.D.; Walker-Smith, J.A.; Campbell, C.A.; Bellamy, D.; Hinsull, S.M. An accurate, long-term, pH-sensitive radio pill for ingestion and implantation. Biotelem. Patient Monit. 1981, 8, 213–227. [Google Scholar]
- Branicki, F.J.; Evans, D.F.; Ogilvie, A.L.; Atkinson, M.; Hardcastle, J.D. Ambulatory monitoring of oesophageal pH in reflux oesophagitis using a portable radiotelemetry system. Gut 1982, 23, 992–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolka, M.A.; Quigley, M.D.; Blanchard, L.A.; Toyota, D.A.; Stephenson, L.A. Validation of a temperature telemetry system during moderate and strenuous exercise. J. Therm. Biol. 1993, 18, 203–210. [Google Scholar] [CrossRef]
- Bongers, C.C.W.G.; Daanen, H.A.M.; Bogerd, C.P.; Hopman, M.T.E.; Eijsvogels, T.M.H. Validity, Reliability, and Inertia of Four Different Temperature Capsule Systems. Med. Sci. Sports Exerc. 2018, 50, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Weitschies, W.; Wedemeyer, R.S.; Kosch, O.; Fach, K.; Nagel, S.; Söderlind, E.; Trahms, L.; Abrahamsson, B.; Mönnikes, H. Impact of the intragastric location of extended release tablets on food interactions. J. Control. Release 2005, 108, 375–385. [Google Scholar] [CrossRef]
- Weitschies, W.; Blume, H.; Monnikes, H. Magnetic marker monitoring: High resolution real-time tracking of oral solid dosage forms in the gastrointestinal tract. Eur. J. Pharm. Biopharm. 2010, 74, 93–101. [Google Scholar] [CrossRef]
- The National Institute for Health and Clinical Excellence (NICE). Wireless Capsule Endoscopy for Investigation of the Small Bowel; National Institute of Clinical Excellence: London, UK, 2004. [Google Scholar]
- Van de Bruaene, C.; De Looze, D.; Hindryckx, P. Small bowel capsule endoscopy: Where are we after almost 15 years of use? World J. Gastrointest. Endosc. 2015, 7, 13–36. [Google Scholar] [CrossRef]
- Diaz Tartera, H.O.; Webb, D.L.; Al-Saffar, A.K.; Halim, M.A.; Lindberg, G.; Sangfelt, P.; Hellström, P.M. Validation of SmartPill(®) wireless motility capsule for gastrointestinal transit time: Intra-subject variability, software accuracy and comparison with video capsule endoscopy. Neurogastroenterol. Motil. 2017, 29, 1–9. [Google Scholar] [CrossRef]
- Schlageter, V.; Besse, P.A.; Popovic, R.S.; Kucera, P. Tracking system with five degrees of freedom using a 2D-array of Hall sensors and a permanent magnet. Sens. Actuators A Phys. 2001, 92, 37–42. [Google Scholar] [CrossRef]
- Hiroz, P.; Schlageter, V.; Givel, J.C.; Kucera, P. Colonic movements in healthy subjects as monitored by a Magnet Tracking System. Neurogastroenterol. Motil. 2009, 21, e838–e857. [Google Scholar] [CrossRef] [Green Version]
- Worsoe, J.; Fynne, L.; Gregersen, T.; Schlageter, V.; Christensen, L.A.; Dahlerup, J.F.; Rijkhoff, N.J.; Laurberg, S.; Krogh, K. Gastric transit and small intestinal transit time and motility assessed by a magnet tracking system. BMC Gastroenterol. 2011, 11, 145. [Google Scholar] [CrossRef] [Green Version]
- Farmer, A.D.; Scott, S.M.; Hobson, A.R. Gastrointestinal motility revisited: The wireless motility capsule. United Eur. Gastroenterol. J. 2013, 1, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Saad, R.J.; Hassler, W.L. A technical review & clinical assessment of the wireless motility capsule. Gastroenterol. Hepatol. 2011, 7, 10. [Google Scholar]
- The National Institute for Health and Clinical Excellence (NICE). Interventional Procedure Overview of Assessing Motility of the Gastrointestinal Tract Using a Wireless Capsule; National Institute of Clinical Excellence: London, UK, 2014. [Google Scholar]
- Maqbool, S.; Parkman, H.P.; Friedenberg, F.K. Wireless capsule motility: Comparison of the SmartPill GI monitoring system with scintigraphy for measuring whole gut transit. Dig. Dis. Sci. 2009, 54, 2167–2174. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Kuo, B.; McCallum, R.W.; Chey, W.D.; DiBaise, J.K.; Hasler, W.L.; Koch, K.L.; Lackner, J.M.; Miller, C.; Saad, R.; et al. Investigation of Colonic and Whole-Gut Transit With Wireless Motility Capsule and Radiopaque Markers in Constipation. Clin. Gastroenterol. Hepatol. 2009, 7, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Richert, H. Entwicklung Eines Magnetischen 3-D-Monitoringsystems am Beispiel der Nichtinvasiven Untersuchung des Menschlichen Gastro-Intestinal-Traktes. PhD Dissertation, Friedrich Schiller Universität, Jena, Germany, 2003. [Google Scholar]
- Matesy. 3D-MAGMA Smart Gastrointestinal Functionality Investigations—Product Overview; Matesy GmbH: Jena, Germany, 2018. [Google Scholar]
- Hocke, M.; Schöne, U.; Richert, H.; Görnert, P.; Keller, J.; Layer, P.; Stallmach, A. Every slow-wave impulse is associated with motor activity of the human stomach. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G709–G716. [Google Scholar] [CrossRef] [Green Version]
- Chongqing Jinshan Science & Technology (Group) Co., Ltd. OMOM HD Capsule Endoscopy Platform. Available online: https://www.jinshangroup.com/solutions/omom-hd-capsule-endoscopy-camera/ (accessed on 20 May 2022).
- Li, C.Y.; Zhang, B.L.; Chen, C.X.; Li, Y.M. OMOM capsule endoscopy in diagnosis of small bowel disease. J. Zhejiang Univ. Sci. B 2008, 9, 857–862. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Velasco, G.; Zamarripa-Mottú, R.A.; Solórzano-Pineda, O.M.; Mascarenhas-Saraiva, M.; Blancas-Valencia, J.M.; Hernández-Mondragón, O.V. Comparison in the diagnostic yield between “Pillcam SB3” capsule endoscopy and “OMOM Smart Capsule 2” in small bowel bleeding. A randomized head-to-head study. Dig. Dis. 2020, 39, 211–216. [Google Scholar] [CrossRef]
- McKenzie, J.E.; Osgood, D.W. Validation of a new telemetric core temperature monitor. J. Therm. Biol. 2004, 29, 605–611. [Google Scholar] [CrossRef]
- Olympus. Smart & Safe EndoCapsule 10. Available online: https://www.olympus.co.uk/medical/rmt/media/en-gb/Content/Content-MSD/Images/SRP-Pages/SRP-Endocapsule10/E0429307_EC-10-Product-Brochure.pdf (accessed on 22 May 2022).
- Cave, D.R.; Fleischer, D.E.; Leighton, J.A.; Faigel, D.O.; Heigh, R.I.; Sharma, V.K.; Gostout, C.J.; Rajan, E.; Mergener, K.; Foley, A.; et al. A multicenter randomized comparison of the Endocapsule and the Pillcam SB. Gastrointest. Endosc. 2008, 68, 487–494. [Google Scholar] [CrossRef]
- Dolak, W.; Kulnigg-Dabsch, S.; Evstatiev, R.; Gasche, C.; Trauner, M.; Püspök, A. A randomized head-to-head study of small-bowel imaging comparing MiroCam and EndoCapsule. Endoscopy 2012, 44, 1012–1020. [Google Scholar] [CrossRef]
- Wang, W.X.; Yan, G.Z.; Sun, F.; Jiang, P.P.; Zhang, W.Q.; Zhang, G.F. A non-invasive method for gastrointestinal parameter monitoring. World J. Gastroenterol. 2005, 11, 521–524. [Google Scholar] [CrossRef] [PubMed]
- CapsoVision. CapsoCam Plus Specifications. Available online: https://capsovision.com/physician-resources/capsocam-plus-specifications/ (accessed on 20 May 2022).
- Pioche, M.; Vanbiervliet, G.; Jacob, P.; Duburque, C.; Gincul, R.; Filoche, B.; Daudet, J.; Filippi, J.; Saurin, J.C. Prospective randomized comparison between axial- and lateral-viewing capsule endoscopy systems in patients with obscure digestive bleeding. Endoscopy 2014, 46, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Zwinger, L.L.; Siegmund, B.; Stroux, A.; Adler, A.; Veltzke-Schlieker, W.; Wentrup, R.; Jürgensen, C.; Wiedenmann, B.; Wiedbrauck, F.; Hollerbach, S.; et al. CapsoCam SV-1 Versus PillCam SB 3 in the Detection of Obscure Gastrointestinal Bleeding: Results of a Prospective Randomized Comparative Multicenter Study. J. Clin. Gastroenterol. 2019, 53, e101–e106. [Google Scholar] [CrossRef] [PubMed]
- Branchi, F.; Ferretti, F.; Orlando, S.; Tontini, G.E.; Penagini, R.; Vecchi, M.; Elli, L. Small-bowel capsule endoscopy in patients with celiac disease, axial versus lateral/panoramic view: Results from a prospective randomized trial. Dig. Endosc. 2020, 32, 778–784. [Google Scholar] [CrossRef]
- Hong, S.N.; Kang, S.H.; Jang, H.J.; Wallace, M.B. Recent Advance in Colon Capsule Endoscopy: What’s New? Clin. Endosc. 2018, 51, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Eliakim, R.; Fireman, Z.; Gralnek, I.M.; Yassin, K.; Waterman, M.; Kopelman, Y.; Lachter, J.; Koslowsky, B.; Adler, S.N. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: Results of the first multicenter, prospective, comparative study. Endoscopy 2006, 38, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Eliakim, R.; Yassin, K.; Niv, Y.; Metzger, Y.; Lachter, J.; Gal, E.; Sapoznikov, B.; Konikoff, F.; Leichtmann, G.; Fireman, Z.; et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy 2009, 41, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Spada, C.; Pasha, S.F.; Gross, S.A.; Leighton, J.A.; Schnoll-Sussman, F.; Correale, L.; González Suárez, B.; Costamagna, G.; Hassan, C. Accuracy of First- and Second-Generation Colon Capsules in Endoscopic Detection of Colorectal Polyps: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1533–1543.e8. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.; Park, J.Y.; Jeong, S.; Kim, Y.H.; Shim, H.B.; Kim, T.S.; Lee, D.H.; Song, S.Y. First clinical trial of the “MiRo” capsule endoscope by using a novel transmission technology: Electric-field propagation. Gastrointest. Endosc. 2009, 69, 253–259. [Google Scholar] [CrossRef]
- Pioche, M.; Gaudin, J.L.; Filoche, B.; Jacob, P.; Lamouliatte, H.; Lapalus, M.G.; Duburque, C.; Chaput, U.; Ben Soussan, E.; Daudet, J.; et al. Prospective, randomized comparison of two small-bowel capsule endoscopy systems in patients with obscure GI bleeding. Gastrointest. Endosc. 2011, 73, 1181–1188. [Google Scholar] [CrossRef]
- Ibekwe, V.C.; Fadda, H.M.; McConnell, E.L.; Khela, M.K.; Evans, D.F.; Basit, A.W. Interplay between intestinal pH, transit time and feed status on the in vivo performance of pH responsive ileo-colonic release systems. Pharm. Res. 2008, 25, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Schlageter, V. Motilis 3D-Transit Gastrointestinal Motility Monitoring System with Software v0.4 Instructions for Use; Motilis: Lausanne, Switzerland, 2014. [Google Scholar]
- Schlageter, V. Motilis 3D-Transit Gastrointestinal Motility Monitoring System Investigator’s Brochure; Motilis: Lausanne, Switzerland, 2014. [Google Scholar]
- Kalsi, G.K.; Gronlund, D.; Martin, J.; Drewes, A.M.; Scott, S.M.; Birch, M.J. Technical report: Inter- and intra-rater reliability of regional gastrointestinal transit times measured using the 3D-Transit electromagnet tracking system. Neurogastroenterol. Motil. 2018, 30, e13396. [Google Scholar] [CrossRef] [PubMed]
- Van der Schaar, P.J.; Dijksman, F.; Shimizu, J.; Wanke, C.; Siersema, P.D. First in Human Study with a Novel Ingestible Electronic Drug Delivery and Monitoring Device: The Intellicap. Gastroenterology 2011, 140, S-766. [Google Scholar] [CrossRef]
- Koziolek, M.; Grimm, M.; Becker, D.; Iordanov, V.; Zou, H.; Shimizu, J.; Wanke, C.; Garbacz, G.; Weitschies, W. Investigation of pH and Temperature Profiles in the GI Tract of Fasted Human Subjects Using the Intellicap((R)) System. J. Pharm. Sci. 2015, 104, 2855–2863. [Google Scholar] [CrossRef]
- Van der Schaar, P.J.; Dijksman, J.F.; Broekhuizen-de Gast, H.; Shimizu, J.; van Lelyveld, N.; Zou, H.; Iordanov, V.; Wanke, C.; Siersema, P.D. A novel ingestible electronic drug delivery and monitoring device. Gastrointest. Endosc. 2013, 78, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Gluck, N.; Shpak, B.; Brun, R.; Rösch, T.; Arber, N.; Moshkowitz, M. A novel prepless X-ray imaging capsule for colon cancer screening. Gut 2016, 65, 371–373. [Google Scholar] [CrossRef] [Green Version]
- Garcia Garcia de Paredes, A.; Gross, S.A.; Hernandez-Lara, A.H.; Hansel, S.L.; Poppers, D.M.; Rajan, E. Colorectal Cancer and Polyp Detection Using a New Preparation-Free, Colon-Scan Capsule: A Pilot Study of Safety and Patient Satisfaction. Dig. Dis. Sci. 2022, 67, 4070–4077. [Google Scholar] [CrossRef]
- Check-Cap Ltd. Check-Cap Announces Positive Final Results from Its Post-CE Approval Study of the C-Scan® System. Available online: https://ir.check-cap.com/2019-07-09-Check-Cap-Announces-Positive-Final-Results-from-Its-Post-CE-Approval-Study-of-the-C-Scan-R-System (accessed on 31 March 2023).
- Kalantar-Zadeh, K.; Berean, K.J.; Ha, N.; Chrimes, A.F.; Xu, K.; Grando, D.; Ou, J.Z.; Pillai, N.; Campbell, J.L.; Brkljača, R.; et al. A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat. Electron. 2018, 1, 79–87. [Google Scholar] [CrossRef]
- Berean, K.J.; Ha, N.; Ou, J.Z.; Chrimes, A.F.; Grando, D.; Yao, C.K.; Muir, J.G.; Ward, S.A.; Burgell, R.E.; Gibson, P.R.; et al. The safety and sensitivity of a telemetric capsule to monitor gastrointestinal hydrogen production in vivo in healthy subjects: A pilot trial comparison to concurrent breath analysis. Aliment. Pharmacol. Ther. 2018, 48, 646–654. [Google Scholar] [CrossRef] [Green Version]
- Thwaites, P.A.; Yao, C.K.; Maggo, J.; John, J.; Chrimes, A.F.; Burgell, R.E.; Muir, J.G.; Parker, F.C.; So, D.; Kalantar-Zadeh, K.; et al. Comparison of gastrointestinal landmarks using the gas-sensing capsule and wireless motility capsule. Aliment. Pharmacol. Ther. 2022, 56, 1337–1348. [Google Scholar] [CrossRef]
- Sarosiek, I.; Espino, K.; Nee, J.; Lembo, A.; Richard, M.W. The MoPill Gastrointestinal Positioning System (GPS): New technology to navigate the alimentary tract highway. In Proceedings of the 19th American Neurogastroenterology and Motility Society Annual Scientific Meeting, Boston, MA, USA, 13–15 August 2021. [Google Scholar]
- Goldstein, J.L.; Eisen, G.M.; Lewis, B.; Gralnek, I.M.; Aisenberg, J.; Bhadra, P.; Berger, M.F. Small bowel mucosal injury is reduced in healthy subjects treated with celecoxib compared with ibuprofen plus omeprazole, as assessed by video capsule endoscopy. Aliment. Pharmacol. Ther. 2007, 25, 1211–1222. [Google Scholar] [CrossRef]
- Malagelada, C.; De Iorio, F.; Azpiroz, F.; Accarino, A.; Segui, S.; Radeva, P.; Malagelada, J.R. New insight into intestinal motor function via noninvasive endoluminal image analysis. Gastroenterology 2008, 135, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Malagelada, C.; De Lorio, F.; Segui, S.; Mendez, S.; Drozdzal, M.; Vitria, J.; Radeva, P.; Santos, J.; Accarino, A.; Malagelada, J.R.; et al. Functional gut disorders or disordered gut function? Small bowel dysmotility evidenced by an original technique. Neurogastroenterol. Motil. 2012, 24, 223–228, e104–225. [Google Scholar] [CrossRef] [PubMed]
- Malagelada, C.; Drozdzal, M.; Seguí, S.; Mendez, S.; Vitrià, J.; Radeva, P.; Santos, J.; Accarino, A.; Malagelada, J.R.; Azpiroz, F. Classification of functional bowel disorders by objective physiological criteria based on endoluminal image analysis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G413–G419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haase, A.M.; Gregersen, T.; Schlageter, V.; Scott, M.S.; Demierre, M.; Kucera, P.; Dahlerup, J.F.; Krogh, K. Pilot study trialling a new ambulatory method for the clinical assessment of regional gastrointestinal transit using multiple electromagnetic capsules. Neurogastroenterol. Motil. 2014, 26, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Mark, E.B.; Nedergaard, R.B.; Hansen, T.M.; Nissen, T.D.; Frøkjaer, J.B.; Scott, S.M.; Krogh, K.; Drewes, A.M. Tapentadol results in less deterioration of gastrointestinal function and symptoms than standard opioid therapy in healthy male volunteers. Neurogastroenterol. Motil. 2021, 33, e14131. [Google Scholar] [CrossRef]
- Monnard, C.R.; Fares, E.J.; Calonne, J.; Miles-Chan, J.L.; Montani, J.P.; Durrer, D.; Schutz, Y.; Dulloo, A.G. Issues in Continuous 24-h Core Body Temperature Monitoring in Humans Using an Ingestible Capsule Telemetric Sensor. Front. Endocrinol. 2017, 8, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, T.; Fujimori, S.; Hayashida, M.; Hanada, R.; Akiyama, J.; Sakamoto, C. Repeatability of small bowel transit time in capsule endoscopy in healthy subjects. Biomed. Mater. Eng. 2018, 29, 839–848. [Google Scholar] [CrossRef]
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Fallingborg, J.; Christensen, L.A.; Ingeman-Nielsen, M.; Jacobsen, B.A.; Abildgaard, K.; Rasmussen, H.H. pH-profile and regional transit times of the normal gut measured by a radiotelemetry device. Aliment. Pharmacol. Ther. 1989, 3, 605–613. [Google Scholar] [CrossRef]
- Koziolek, M.; Schneider, F.; Grimm, M.; Modeβ, C.; Seekamp, A.; Roustom, T.; Siegmund, W.; Weitschies, W. Intragastric pH and pressure profiles after intake of the high-caloric, high-fat meal as used for food effect studies. J. Control. Release 2015, 220, 71–78. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, J.; Murphy, C.L.; Barry, L.; Shanahan, F.; Buckley, M. Defining gastrointestinal transit time using video capsule endoscopy: A study of healthy subjects. Endosc. Int. Open 2020, 8, E396–E400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooks, S.B., 3rd; Rutland, T.J.; Di Palma, J.A. Lubiprostone neither decreases gastric and small-bowel transit time nor improves visualization of small bowel for capsule endoscopy: A double-blind, placebo-controlled study. Gastrointest. Endosc. 2009, 70, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Jianqin, S.; Leiming, X.; Lu, X.; Yelland, G.W.; Ni, J.; Clarke, A.J. Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr. J. 2016, 15, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creedon, A.C.; Dimidi, E.; Hung, E.S.; Rossi, M.; Probert, C.; Grassby, T.; Miguens-Blanco, J.; Marchesi, J.R.; Scott, S.M.; Berry, S.E.; et al. The impact of almonds and almond processing on gastrointestinal physiology, luminal microbiology, and gastrointestinal symptoms: A randomized controlled trial and mastication study. Am. J. Clin. Nutr. 2022, 116, 1790–1804. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, S.; Gudis, K.; Takahashi, Y.; Seo, T.; Yamada, Y.; Ehara, A.; Kobayashi, T.; Mitsui, K.; Yonezawa, M.; Tanaka, S.; et al. Distribution of small intestinal mucosal injuries as a result of NSAID administration. Eur. J. Clin. Investig. 2010, 40, 504–510. [Google Scholar] [CrossRef]
- Sangnes, D.A.; Lundervold, K.; Bekkelund, M.; von Volkmann, H.L.; Berentsen, B.; Gilja, O.H.; Dimcevski, G.; Søfteland, E. Gastrointestinal transit and contractility in diabetic constipation: A wireless motility capsule study on diabetes patients and healthy controls. United Eur. Gastroenterol. J. 2021, 9, 1168–1177. [Google Scholar] [CrossRef]
- Von Volkmann, H.L.; Brønstad, I.; Gilja, O.H.; Tronstad, R.R.; Sangnes, D.A.; Nortvedt, R.; Hausken, T.; Dimcevski, G.; Fiskerstrand, T.; Nylund, K. Prolonged intestinal transit and diarrhea in patients with an activating GUCY2C mutation. PLoS ONE 2017, 12, e0185496. [Google Scholar] [CrossRef] [Green Version]
- Fujimori, S.; Seo, T.; Gudis, K.; Ehara, A.; Kobayashi, T.; Mitsui, K.; Yonezawa, M.; Tanaka, S.; Tatsuguchi, A.; Sakamoto, C. Prevention of nonsteroidal anti-inflammatory drug-induced small-intestinal injury by prostaglandin: A pilot randomized controlled trial evaluated by capsule endoscopy. Gastrointest. Endosc. 2009, 69, 1339–1346. [Google Scholar] [CrossRef]
- Brewer, C.; Harrower, M.; Sheesley, B.; Woodruff, A.; Heyman, D. ColorBrewer 2.0. Available online: https://colorbrewer2.org/# (accessed on 1 July 2021).
- Sharif, H.; Devadason, D.; Abrehart, N.; Stevenson, R.; Marciani, L. Imaging Measurement of Whole Gut Transit Time in Paediatric and Adult Functional Gastrointestinal Disorders: A Systematic Review and Narrative Synthesis. Diagnostics 2019, 9, 221. [Google Scholar] [CrossRef] [Green Version]
- Abuhelwa, A.Y.; Foster, D.J.R.; Upton, R.N. A Quantitative Review and Meta-models of the Variability and Factors Affecting Oral Drug Absorption-Part II: Gastrointestinal Transit Time. AAPS J. 2016, 18, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Ewe, K.; Press, A.G.; Bollen, S.; Schuhn, I. Gastric emptying of indigestible tablets in relation to composition and time of ingestion of meals studied by metal detector. Dig. Dis. Sci. 1991, 36, 146–152. [Google Scholar] [CrossRef]
- Cassilly, D.; Kantor, S.; Knight, L.C.; Maurer, A.H.; Fisher, R.S.; Semler, J.; Parkman, H.P. Gastric emptying of a non-digestible solid: Assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol. Motil. 2008, 20, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Deloose, E.; Janssen, P.; Depoortere, I.; Tack, J. The migrating motor complex: Control mechanisms and its role in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Guo, Y.; Mashimo, H. Advances in the physiology of gastric emptying. Neurogastroenterol. Motil. 2019, 31, e13546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, A.; Asahina, M.; Ishikawa, C.; Asahina, K.M.; Honma, K.; Fukutake, T.; Hattori, T. Impaired circadian rhythm of gastric myoelectrical activity in patients with multiple system atrophy. Clin. Auton. Res. 2005, 15, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Code, C.F.; Marlett, J.A. The interdigestive myo-electric complex of the stomach and small bowel of dogs. J. Physiol. 1975, 246, 289–309. [Google Scholar] [CrossRef]
- Fadda, H.M.; McConnell, E.L.; Short, M.D.; Basit, A.W. Meal-induced acceleration of tablet transit through the human small intestine. Pharm. Res. 2009, 26, 356–360. [Google Scholar] [CrossRef]
- Keller, J.; Bassotti, G.; Clarke, J.; Dinning, P.; Fox, M.; Grover, M.; Hellstrom, P.M.; Ke, M.; Layer, P.; Malagelada, C.; et al. Expert consensus document: Advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 291–308. [Google Scholar] [CrossRef] [Green Version]
- Asnicar, F.; Leeming, E.R.; Dimidi, E.; Mazidi, M.; Franks, P.W.; Al Khatib, H.; Valdes, A.M.; Davies, R.; Bakker, E.; Francis, L.; et al. Blue poo: Impact of gut transit time on the gut microbiome using a novel marker. Gut 2021, 70, 1665–1674. [Google Scholar] [CrossRef]
- Heaton, K.W.; Radvan, J.; Cripps, H.; Mountford, R.A.; Braddon, F.E.; Hughes, A.O. Defecation frequency and timing, and stool form in the general population: A prospective study. Gut 1992, 33, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.S.; Coss-Adame, E.; Valestin, J.; Mysore, K. Evaluation of constipation in older adults: Radioopaque markers (ROMs) versus wireless motility capsule (WMC). Arch. Gerontol. Geriatr. 2012, 55, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Adler, S.N.; Aisenberg, J.; Burch, W.C., Jr.; Carretero, C.; Chowers, Y.; Fein, S.A.; Fern, S.E.; Fernandez-Urien Sainz, I.; Fich, A.; et al. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology 2015, 148, 948–957.e2. [Google Scholar] [CrossRef] [PubMed]
- Southwell, B.R.; Clarke, M.C.; Sutcliffe, J.; Hutson, J.M. Colonic transit studies: Normal values for adults and children with comparison of radiological and scintigraphic methods. Pediatr. Surg. Int. 2009, 25, 559–572. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Participants |
|
|
| Interventions |
| Use of:
|
| Any studies that performed extensive bowel cleansing prior to capsule ingestion or involved an intervention or treatment that could affect GI transit times | ||
| Comparisons | Not applicable | Not applicable |
| Outcomes |
| Not applicable |
| Study designs |
|
|
| Device | Year Introduced | Sensing Capabilities/Indications for Use | System Components | System Component Dimensions | Measurement Range and Accuracy | Battery Life | Transit Time Measurement Capabilities | Measurement Validation | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GET | SITT | CTT | WGTT | ||||||||
| Heidelberg Capsule (Heidelberg Medical, Germany) [8,9] | ~1960s | Sensor: gut pH; Commercially available. | Ingestible pH capsule; Transceiver; Interface Module; pH capsule locator; Dedicated software. | Capsule Ø 8 mm, length 21 mm | pH measurement range: 1–8 pH units pH accuracy: ±0.5 pH units | Transceiver: 12–14 h | ✓ | Acid values obtained from the capsule were compared against tube aspiration and showed acceptable correlation [10]. No comparative transit time studies. | |||
| Radiotelemetry Capsule (RTC) (Remote Control Systems Ltd., Consett, UK) * [11,12] | 1981 | Sensor: gut pH; Research use only. | Ingestible pH capsule; Portable solid-state receiver. | Capsule Ø 7.6 mm, length 26 mm Solid state receiver: 400 g | pH measurement range: 1–10 pH units pH accuracy: ±0.2 pH units | 24 h (Fs = 6 s) or up to 48 h (Fs = 12 s) | ✓ | ✓ | No comparative transit time studies. | ||
| CorTemp® (HQ, Inc., Palmetto, FL, USA) * [13,14] | ~1988 | Sensor: core body temperature; Commercially available. | Ingestible capsule; Data recorder; Dedicated software (CorTrack® II). | Capsule Ø 10.9 mm, length 22.4 mm, weight 2.8 g | Operating range: 30 °C to 45 °C Accuracy: 0.27 °C | 7–10 days | ✓ | Good reliability when compared against oesophageal and rectal temperatures, however capsule mobility results in measurement variability [13]. Water bath validation shows excellent validity and test-retest reliability, after removal of outlier [14]. No comparative transit time studies. | |||
| Magnetic Marker Monitoring System (Department of Biopharmaceutics and Pharmaceutical Technology, University of Greifswald, Greifswald, Germany) [15,16] | 1994 | Sensor: magnetic; GI localisation for real-time tracking of dosage forms; Research use only. | Ingestible magnetised tablets containing drug and ferromagnetic black iron oxide; Biomagnetic measurement device containing SQUIDs. | Tablet Ø 11 mm Biomagnetic measurement device coverage range: Ø 230 mm | High spatial and temporal resolution in the range of 1 mm | N/A Mains powered system | ✓ | ✓ | Experimental set-ups using test objects of known dimensions demonstrates high spatial and temporal resolution [16]. No comparative transit time studies. | ||
| PillCam™ SB (Medtronic Inc., Minneapolis, MN, USA) * [17,18,19] | 2000 | Sensor: video camera; Indicated for obscure GI bleeding and the diagnosis and investigation of Crohn’s disease [17]; Commercially available. | Ingestible video capsule; Sensor belt and sensor array; Data recorder; Dedicated software (PillCamTM software v9) | Capsule Ø 11.4 mm, length 26.2 mm, weight 3 g | Minimum size of detection—0.07 mm Image resolution—340 × 340 Frame rate: 2–6 fps Field of view: 156° | 8–12 h | ✓ | ✓ | PillCam yields shorter GET and SITT than WMC [19]. | ||
| Motility Tracking System (Motilis Medica SA, Lausanne, Switzerland) [20,21,22] | 2002 | Sensor: magnetic. Regional GI transit times and motility patterns e.g., regional contraction frequencies, velocities, segment lengths and direction of movement GI localisation for real-time tracking; Research use only. | Ingestible magnetic capsule (permanent magnet); 4 × 4 matrix of sensors; Dedicated software (MTS_Record) | Capsule Ø 6 mm, length 15 mm | Position accuracy: ±5% | Unknown | ✓ | ✓ | Good agreement seen in GET and SITT values obtained from the MTS capsule and PillCam [22]. | ||
| WMC (SmartPill™) (Medtronic Inc., Minneapolis, MN, USA) * [23,24] | 2003 | Sensor: gut pH, temperature and pressure. Indicated for the evaluation of GI motility disorders e.g., suspected delayed gastric emptying and differentiation between normal and slow transit constipation [25]; Commercially available. | Ingestible capsule; Data receiver; Dedicated software (MotiliGITM Software) | Capsule Ø 26.8 mm, length 11.7 mm, weight 4.5 g Receiver: approx. 150 mm × 100 mm × 38 mm | Pressure range: −0–350 mmHg Pressure accuracy: ±5 mmHg below 100 mmHg Temperature range: 25–49 °C Temperature accuracy: ±0.5 °C pH measurement range: 0.05–9.0 pH units pH accuracy: ±0.5 pH units | Capsule and Data receiver, >5 days | ✓ | ✓ | ✓ | ✓ | Several transit studies have shown good agreement between WMC and ROMs or scintigraphy [26,27]. |
| 3D-MAGMA (Matesy GmbH, Jena, Germany) * [28,29] | 2003 | Sensor: magnetic; Real-time tracking of magnetic markers for the measurement of gut contraction frequencies and power, transit times marker progression paths and velocities; Commercially available. | Ingestible permanent magnetic capsule; Sensor system containing 27 magnetic field sensors. Dedicated software | Capsule Ø 6 mm, length 16 mm, density ~<1.5 g/cm3 | Realtime position tracking accuracy: 3 mm | N/A Mains-powered system | ✓ | Strong linear correlation between 3D-MAGMA and Electrogastrography for the measurement of gastric slow waves [30]. No comparative transit time studies found | |||
| OMOM® (Jinshan Science and Technology Company, Chongqing, China) * [31,32] | 2004 | Sensor: video camera; For small bowel evaluation. | Ingestible video capsule; Portable image recorder. Dedicated software (SmartScan, SmartView, SmartFinding, Vue Smart) | Capsule Ø 11 mm, length 25.4 mm, weight: 3 g | Depth of field: 0–50 mm Minimum size of detection—0.1 mm Image resolution—512 × 512 Frame rate: 2–10 fps Field of view: 172° | 12 h | ✓ | ✓ | ✓ | Diagnostic yield, functionality and SITT of OMOM compared against PillCam SB3. No statistically significant difference found between the two systems [33]. | |
| VitalSense® (Philips Respironics, OR, USA) [14,34] | 2004 | Sensor: core body temperature; Commercially available. | Ingestible capsule; Data recorder; Dedicated software (Equivital Manager v1.2.39.4600). | Capsule Ø 8.7 mm, length 23 mm, weight: 1.5 g | Operating range: −10 °C to 60 °C Accuracy: 0.17 °C | 10 days | ✓ | No significant differences between capsule and rectal measure temperatures [34]. Good validity and test-retest reliability in water bath, after removal of outliers [14]. No comparative transit time studies. | |||
| EndoCapsule (Olympus Inc., Tokyo, Japan) [35] | 2005 | Sensor: video camera; visualisation of small intestinal mucosa; Commercially available. | Ingestible capsule; Recorder; Battery pack; Antenna Unit; Capsule activator; Recorder; antenna holder and cradle; Dedicated software (ENDOCAPSULE SOFTWARE 10) | Capsule Ø 11 mm, length 26 mm, weight: 3.3 g Recorder: 87 mm × 154 mm × 33 mm, weight: 320 g Battery pack: 70 mm × 10 mm × 55 mm, weight: 70 g Antenna: 87 mm × 51 mm × 15 mm, weight: 150 g | Field of view: 160° Depth of field: 0–20 mm Frames per second: 2 | Capsule: 12 h Recorder: 12 h | ✓ | ✓ | No significant difference in mean SITT between EndoCapsule and PillCam SB in patients with OGIB [36]. Similarly, no statistically significant difference in GET and SITT between EndoCapsule and MiroCam as measured in patients referred for VCE [37]. | ||
| Experimental system Institute of Precision Engineering and Intelligent Microsystem, Shanghai Jiaotong University, Shanghai, China [38] | 2005 | Sensor: gut pH, temperature and pressure; Research use only. | 2 indigestible biotelemetry capsules:
Ultrasonic electrode waistcoat; Dedicated software. | Capsule Ø 10 mm, length 21.1 mm, weight: 2.9 g pH capsule: Ø 10 mm, length 24 mm, weight: 5.2 g | Pressure range: −60–200 mmHg Pressure accuracy: 1% Temperature range: 34–42 °C Temperature accuracy: ±0.2 °C pH measurement range: 1–13 pH units pH accuracy: ±0.2 pH units | Unknown | ✓ | Laboratory tests performed by a test house, measuring against gauge data, verifying feasibility and functionality [38]. No comparative transit time studies. | |||
| CapsoCam® (CapsoVision, Cupertino, CA, USA) [39] | 2006 | Sensor: video camera; Provides a 360° panoramic view of the small bowel mucosa; Commercially available. | Ingestible capsule with on-board data storage capabilities, avoiding the need for external recording equipment. Dedicated software (CapsoVision) | CapsoCam Plus capsule: Ø 11 mm, length 31 mm Weight: 4 g | Image resolution 221,184 Max frame rate: 20 fps Field of view: 360° Depth of view: 0–18 mm No. of cameras: 4 | 15 h | ✓ | ✓ | Several patient studies performed comparing diagnostic findings of CapsoCam against PillCam SB. No statistically significant differences found in GET and SITT between the two systems [40,41,42]. | ||
| PillCam™ Colon (Medtronic Inc., Minneapolis, MN, USA) [43] | 2006 | Sensor: video camera; colon capsule endoscopy for polyp detection, diagnosing inflammatory bowel disease and colorectal cancer screening; Commercially available. | Ingestible video capsule; Sensor belt and sensor array; Data recorder; Dedicated software (PillCamTM software v9) | Capsule Ø 11.6 mm, length 32.3 mm, weight: 2.9 g | Field of View: 172°; Minimum detectable object: at least 0.1 mm; Frame rate: 4–35 fps | 10 h | ✓ | ✓ | Validated against colonoscopy for detection of colorectal polyps/other diseases [44,45,46]. No comparative transit time studies found. | ||
| MiroCam® (IntroMedic Co., Seoul, Republic of Korea) [18,47] | 2009 | Sensor: video camera; exploration of entire small bowel; Commercially available. | Ingestible video capsule; Sensor pads (images transmitted via Human Body Communication); Receiver; Dedicated software (MiroViewTM software) | Capsule Ø 10.8 mm, length 24 mm, weight: 3.3 g | Image resolution—320 × 320 Frames per second: 2 Field of view: 150° | 9–11 h | ✓ | ✓ | Several studies compared the diagnostic yield of the MiroCam against other capsule endoscopy systems e.g., PillCam [48] or EndoCapsule [37] but no comparison of transit times. | ||
| Bravo™ pH capsule (Medtronic Inc., Minneapolis, MN, USA) [49] | 2011 | Sensor: oesophageal pH; Indicated for gastro-oesophageal reflux monitoring; Commercially available. | Ingestible capsule; Data recorder; Dedicated software (BravoTM Reflux Recorder) | Capsule 5 mm × 6 mm × 25 mm, weight: 1.5 g | pH measurement range: 0.5–9.0 pH units | Up to 96 h | ✓ | ✓ | Transit times obtained from capsule compared against those obtained from radiolabelled tablets. Some differences possibly due to size differences between capsule and tables [49]. | ||
| 3D-Transit (Motilis Medica SA, Lausanne, Switzerland) * [50,51] | 2012 | Sensor: electromagnetic; Regional and segmental GI transit times and motility patterns e.g., regional contraction frequencies, velocities, segment lengths and direction of movement GI localisation for real-time tracking. Research use only. | Ingestible electromagnetic capsule; Detector plate and power supply; Respiration measurement belt; Dedicated software (MTS2 software) | Capsule Ø 8.3 mm, length 23 mm, weight: 1.8 g Detector plate: 160 mm × 160 mm × 11 mm; weight: 145 g | Detector range: 4–40 cm (not indicated for abdominal diameter > 140 cm) Absolute position inaccuracy: 10% of the distance between the capsule and the detector at the maximum. | Capsule: 60 h (at 10 Hz) and 120 h (at 5 Hz) | ✓ | ✓ | ✓ | ✓ | No direct transit time comparison studies against other methods however, good inter and intra-rater reliability of measurements seen [5,52]. |
| IntelliCap® (Medimetrics, Eindhoven, The Netherlands) * [53,54] | 2013 | Sensor: gut pH and temperature sensing; For electronic drug delivery and monitoring; Commercially available. | Ingestible capsule; Start-up unit to program and activate capsule; Portable recording unit that transmits data to a PC; Dedicated software | Capsule Ø 11 mm, length 27 mm | Relative pH accuracy: ±0.3 pH units Relative temperature accuracy: ±0.1 °C | Capsule battery lasts at least 48 h | ✓ | ✓ | ✓ | ✓ | Capsule localisation compared to scintigraphy and shown to correlate well [55]. No comparative transit time studies found. |
| C-scan® system (Check-Cap Inc., Isfiya, Israel) [56,57] | 2014 | Sensor: ultra-low dose X-ray source (Tungsten 181 Radioisotope); temperature, pressure and radio frequency signalling; For polyp detection; Commercially available. | Ingestible Capsule (C-Scan® Cap); Recorder (C-Scan ®Track); Dedicated workstation; Dedicated software (C-Scan® View). | Capsule Ø 11.6 mm, length 34 mm | Capsule position and orientation accuracy in colon: ±1 cm | Capsule: battery lasts 100 h | ✓ | 76% sensitivity and 82% specificity for the detection of precancerous polyps when compared to fecal immunochemical test [58]. | |||
| MyTemp (MyTemp, Nijmegen, The Netherlands) [14] | ~2016 | Sensor: core body temperature; Research use only. | Ingestible capsule; Copper-wired waistband; Dedicated software (myTemp manager v01.08). | Capsule Ø 8 mm, length 20 mm, weight: 1.3 g | Operating range: 30 °C to 45 °C Accuracy: ±0.001 °C | Infinite (no battery—self-induction) | ✓ | Water bath validation shows excellent validity and test-retest reliability, after removal of outliers [14]. No comparative transit time studies. | |||
| e-Celsius® (BodyCap, Caen, France) [14] | CE-marked version introduced in 2017 | Sensor: core body temperature; Commercially available. | Ingestible capsule; External recorder; Dedicated software (e-Performance manager v01.01.00.0C). | Capsule Ø 8.9 mm, length 17.7 mm, weight: 1.7 g | Operating range: −0 °C to 50 °C Accuracy: ±0.23 °C | 20 days | ✓ | Water bath validation shows excellent validity and test-retest reliability, after removal of outliers [14]. No comparative transit time studies. | |||
| Gas sensing capsule (Atmo Biosciences, Box Hill, VIC, Australia) * [59,60,61] | 2018 | Sensors: temperature, relative humidity, hydrogen and carbon dioxide concentration, along with concentrations of total relative volatile organic compounds, capsule orientation and changes in the physical electromagnetic properties of the capsule’s environment [61]. Measures gas concentrations in aerobic and anaerobic conditions within the gut; Research use only. | Ingestible gas sensing capsule; Handheld receiver; Mobile phone software application. | Capsule Ø 11 mm, length 28 mm | Gas sensing accuracy of earlier versions of the capsule: Hydrogen and oxygen better than 0.2% and Carbon dioxide—1% [59]. Sensor range and accuracy of latest version not currently in publication | Capsule: 4 days Temperature sensor and transmission circuitry~30 days | ✓ | ✓ | ✓ | ✓ | Anatomical landmarks as determined using the gas sensing capsule was validated by concurrent (tandem) ingestion of the WMC—good agreement in transit time measurements [61]. |
| MoPillTM (Texas Tech University Health Sciences Center, Lubbock, TX, USA) [62] | 2021 | Sensor: radio frequency (RF) signalling RF position system; Regional and segmental GI transit times GI localisation for real-time tracking; Research use only. | Ingestible capsule; 4 adhesive sensors—2 for abdomen and 2 for back; Recorder; Dedicated software. | Capsule Ø 12 mm, length 20 mm Adhesive sensors: 60 mm × 55 mm | Localisation accuracy range: 9–94 mm | Unknown | ✓ | ✓ | ✓ | ✓ | Capsule location validated using X-ray imaging [62]. |
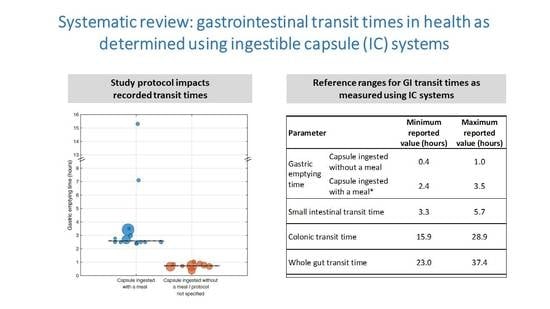
| Parameter | Minimum Reported Value (Hours) | Maximum Reported Value (Hours) | |
|---|---|---|---|
| Gastric emptying time | Capsule ingested without a meal | 0.4 | 1.0 |
| Capsule ingested with a meal * | 2.4 | 3.5 | |
| Small intestinal transit time | 3.3 | 5.7 | |
| Colonic transit time | 15.9 | 28.9 | |
| Whole gut transit time | 23.0 | 37.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandhra, G.K.; Chaichanavichkij, P.; Birch, M.; Scott, S.M. Gastrointestinal Transit Times in Health as Determined Using Ingestible Capsule Systems: A Systematic Review. J. Clin. Med. 2023, 12, 5272. https://doi.org/10.3390/jcm12165272
Nandhra GK, Chaichanavichkij P, Birch M, Scott SM. Gastrointestinal Transit Times in Health as Determined Using Ingestible Capsule Systems: A Systematic Review. Journal of Clinical Medicine. 2023; 12(16):5272. https://doi.org/10.3390/jcm12165272
Chicago/Turabian StyleNandhra, Gursharan Kaur, Phakanant Chaichanavichkij, Malcolm Birch, and S. Mark Scott. 2023. "Gastrointestinal Transit Times in Health as Determined Using Ingestible Capsule Systems: A Systematic Review" Journal of Clinical Medicine 12, no. 16: 5272. https://doi.org/10.3390/jcm12165272