Mitochondrial Aconitase Enzymatic Activity: A Potential Long-Term Survival Biomarker in the Blood of ALS Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Clinical Variables
2.3. Biochemical Procedures
2.4. Statistical Analyses
3. Results
3.1. Demographic and Clinical Variables (Table 1)
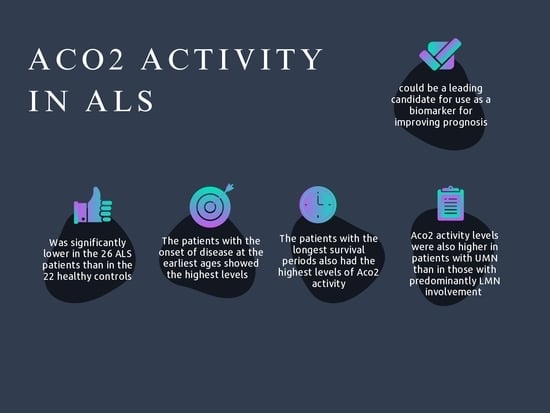
3.2. Aco2 Activity in ALS vs. Controls
3.3. Aco2 Activity and ALS Severity
3.4. ROC Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cook, C.; Petrucelli, L. Genetic Convergence Brings Clarity to the Enigmatic Red Line in ALS. Neuron 2019, 101, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Van Broeckhoven, C.; van der Zee, J. ALS Genes in the Genomic Era and their Implications for FTD. Trends Genet. 2018, 34, 404–423. [Google Scholar] [CrossRef]
- Westeneng, H.J.; Debray, T.P.A.; Visser, A.E.; van Eijk, R.P.A.; Rooney, J.P.K.; Calvo, A.; Martin, S.; McDermott, C.J.; Thompson, A.G.; Pinto, S.; et al. Prognosis for patients with amyotrophic lateral sclerosis: Development and validation of a personalised prediction model. Lancet Neurol. 2018, 17, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Agar, J.; Durham, H. Relevance of oxidative injury in the pathogenesis of motor neuron diseases. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2003, 4, 232–242. [Google Scholar] [CrossRef]
- Johnston, J.A.; Dalton, M.J.; Gurney, M.E.; Kopito, R.R. Formation of high molecular weight complexes of mutant Cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2000, 97, 12571–12576. [Google Scholar] [CrossRef]
- Warita, H.; Hayashi, T.; Murakami, T.; Manabe, Y.; Abe, K. Oxidative damage to mitochondrial DNA in spinal motoneurons of transgenic ALS mice. Brain Res. Mol. 2001, 89, 147–152. [Google Scholar] [CrossRef]
- Babu, G.N.; Kumar, A.; Chandra, R.; Puri, S.K.; Singh, R.L.; Kalita, J.; Misra, U.K. Oxidant-antioxidant imbalance in the erythrocytes of sporadic amyotrophic lateral sclerosis patients correlates with the progression of disease. Neurochem. Int. 2008, 52, 1284–1289. [Google Scholar] [CrossRef]
- Liu, R.; Li, B.; Flanagan, S.W.; Oberley, L.W.; Gozal, D.; Qiu, M. Increased mitochondrial antioxidative activity or decreased oxygen free radical propagation prevent mutant SOD1-mediated motor neuron cell death and increase amyotrophic lateral sclerosis-like transgenic mouse survival. J. Neurochem. 2002, 80, 488–500. [Google Scholar] [CrossRef]
- Hervias, I.; Beal, M.F.; Manfredi, G. Mitochondrial dysfunction and amyotrophic lateral sclerosis. Muscle Nerve 2006, 33, 598–608. [Google Scholar] [CrossRef]
- Laferriere, F.; Polymenidou, M. Advances and challenges in understanding the multifaceted pathogenesis of amyotrophic lateral sclerosis. Swiss Med. Wkly. 2015, 145, w14054. [Google Scholar] [CrossRef] [PubMed]
- Sever, B.; Ciftci, H.; DeMirci, H.; Sever, H.; Ocak, F.; Yulug, B.; Tateishi, H.; Tateishi, T.; Otsuka, M.; Fujita, M.; et al. Comprehensive Research on Past and Future Therapeutic Strategies Devoted to Treatment of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2022, 23, 2400. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, H.M.; Dimachkie, M.M.; Agbas, A. Blood-based Biomarkers for Amyotrophic Lateral Sclerosis. In Amyotrophic Lateral Sclerosis; Araki, T., Ed.; Exon Publications: Brisbane, Australia, 2021. [Google Scholar]
- Kadena, K.; Vlamos, P. The Importance of Diagnostic and Prognostic Biomarker Identification and Classification towards Understanding ALS Pathogenesis. Adv. Exp. Med. Biol. 2021, 1339, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Khodagholi, F.; Shaerzadeh, F.; Montazeri, F. Mitochondrial Aconitase in Neurodegenerative Disorders: Role of a Metabolism- related Molecule in Neurodegeneration. Curr. Drug Targets 2018, 19, 973–985. [Google Scholar] [CrossRef]
- Goutman, S.A.; Hardiman, O.; Al-Chalabi, A.; Chió, A.; Savelieff, M.G.; Kiernan, M.C.; Feldman, E.L. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. 2022, 21, 480–493. [Google Scholar] [CrossRef]
- Verber, N.S.; Shepheard, S.R.; Sassani, M.; McDonough, H.E.; Moore, S.A.; Alix, J.J.P.; Wilkinson, I.D.; Jenkins, T.M.; Shaw, P.J. Biomarkers in motor neuron disease: A state of the art review. Front. Neurol. 2019, 10, 291. [Google Scholar] [CrossRef]
- Dreger, M.; Steinbach, R.; Otto, M.; Turner, M.R.; Grosskreutz, J. Cerebrospinal fluid biomarkers of disease activity and progression in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 422–435. [Google Scholar] [CrossRef]
- Araujo, B.G.; Souza, E.; Silva, L.F.; de Barros Torresi, J.L.; Siena, A.; Valerio, B.C.O.; Brito, M.D.; Rosenstock, T.R. Decreased Mitochondrial Function, Biogenesis, and Degradation in Peripheral Blood Mononuclear Cells from Amyotrophic Lateral Sclerosis Patients as a Potential Tool for Biomarker Research. Mol. Neurobiol. 2020, 57, 5084–5102. [Google Scholar] [CrossRef]
- Turner, M.R.; Swash, M. The expanding syndrome of amyotrophic lateral sclerosis: A clinical and molecular odyssey. J. Neurol. Neurosurg. Psychiatry 2015, 86, 667–673. [Google Scholar] [CrossRef]
- Lushchak, O.V.; Piroddi, M.; Galli, F.; Lushchak, V.I. Aconitase post-translational modification as a key in linkage between Krebs cycle, iron homeostasis, redox signaling, and metabolism of reactive oxygen species. Redox Rep. 2013, 19, 8–15. [Google Scholar] [CrossRef]
- Halliwell, B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Levy, G.; Thompson, J.L.P.; Delbene, M.L.; Battista, V.; Gordon, P.H.; Rowland, L.P.; Levin, B.; Mitsumoto, H. The ALSFRSr predicts survival time in an ALS clinic population. Neurology 2005, 64, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Magnus, T.; Beck, M.; Giess, R.; Puls, I.; Naumann, M.; Toyka, K.V. Disease progression in amyotrophic lateral sclerosis: Predictors of survival. Muscle Nerve 2002, 25, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Larrode-Pellicer, P.; Alberti-González, O.; Iñiguez-Martínez, C.; Pérez-Lázaro, C.; López del Val, L.J. Pronostic factors and survival in motor neuron disease. Neurologia 2007, 22, 362–367. [Google Scholar]
- Dupuis, L.; Corcia, P.; Fergani, A.; De Aguilar, J.-L.G.; Bonnefont-Rousselot, D.; Bittar, R.; Seilhean, D.; Hauw, J.-J.; Lacomblez, L.; Loeffler, J.-P.; et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 2008, 70, 1004–1009. [Google Scholar] [CrossRef]
- Chiò, A.; Calvo, A.; Ilardi, A.; Cavallo, E.; Moglia, C.; Mutani, R.; Palmo, A.; Galletti, R.; Marinou, K.; Papetti, L.; et al. Lower serum lipid levels are related to respiratory impairment in patients with ALS. Neurology 2009, 73, 1681–1685. [Google Scholar] [CrossRef]
- Goodall, E.F.; Haque, M.S.; Morrison, K.E. Increased serum ferritin levels in amyotrophic lateral sclerosis (ALS) patients. J. Neurol. 2008, 255, 1652–1656. [Google Scholar] [CrossRef]
- Mitchell, R.M.; Simmons, Z.; Beard, J.L.; Stephens, H.E.; Connor, J.R. Plasma biomarkers associated with ALS and their relationship to iron homeostasis. Muscle Nerve 2010, 42, 95–103. [Google Scholar] [CrossRef]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Pallotti, F.; Lenaz, G. Isolation and subfractionation of mitochondria from animal cells and tissue culture lines. Methods Cell Biol. 2001, 65, 1–35. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Drapier, J.C.; Hibbs, J.B., Jr. Aconitases: A class of metalloproteins highly sensitive to nitric oxide synthesis. Methods Enzymol. 1996, 269, 26–36. [Google Scholar] [PubMed]
- Obuchowski, N.A.; Bullen, J.A. Receiver operating characteristic (ROC) curves: Review of methods with applications in diagnostic medicine. Phys. Med. Biol. 2018, 63, 07TR01. [Google Scholar] [CrossRef] [PubMed]
- Sturmey, E.; Malaspina, A. Blood biomarkers in ALS: Challenges, applications and novel frontiers. Acta Neurol. Scand. 2022, 146, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Moglia, C.; Canosa, A.; Manera, U.; D’Ovidio, F.; Vasta, R.; Grassano, M.; Brunetti, M.; Barberis, M.; Corrado, L.; et al. ALS phenotype is influenced by age, sex, and genetics: A population-based study. Neurology 2020, 94, e802–e810. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.D.; Little, J.; Gomes, J.; Cashman, N.R.; Krewski, D. Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology 2017, 61, 101–130. [Google Scholar] [CrossRef] [PubMed]
- Su, W.M.; Cheng, Y.F.; Jiang, Z.; Duan, Q.Q.; Yang, T.M.; Shang, H.F.; Chen, Y.P. Predictors of survival in patients with amyotrophic lateral sclerosis: A large meta-analysis. eBioMedicine 2021, 74, 103732. [Google Scholar] [CrossRef]
- Cova, E.; Bongioanni, P.; Cereda, C.; Metelli, M.R.; Salvaneschi, L.; Bernuzzi, S.; Guareschi, S.; Rossi, B.; Ceroni, M. Time course of oxidant markers and antioxidant defenses in subgroups of amyotrophic lateral sclerosis patients. Neurochem. Int. 2010, 56, 687–693. [Google Scholar] [CrossRef]
- Völkel, H.; Selzle, M.; Walk, T.; Jung, G.; Link, J.; Ludolph, A.C.; Reuter, A. Reduced reactivation rate in mutant CuZnSOD and progression rate of amyotrophic lateral sclerosis. Eur. J. Neurol. 2004, 11, 397–404. [Google Scholar] [CrossRef]
- Yang, X.; Ji, Y.; Wang, W.; Zhang, L.; Chen, Z.; Yu, M.; Shen, Y.; Ding, F.; Gu, X.; Sun, H. Amyotrophic Lateral Sclerosis: Molecular Mechanisms, Biomarkers, and Therapeutic Strategies. Antioxidants 2021, 10, 1012. [Google Scholar] [CrossRef]
- Lu, C.H.; Macdonald-Wallis, C.; Gray, E.; Pearce, N.; Petzold, A.; Norgren, N.; Giovannoni, G.; Fratta, P.; Sidle, K.; Fish, M.; et al. Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015, 84, 2247–2257, Erratum in Neurology 2015, 85, 921. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Qin, X.; Chang, X.; Wang, H.; Guo, J.; Zhang, W. Neurofilament markers in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. J. Cell. Mol. Med. 2022, 26, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Verde, F.; Otto, M.; Silani, V. Neurofilament Light Chain as Biomarker for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Front. Neurosci. 2021, 15, 679199. [Google Scholar] [CrossRef] [PubMed]
- Nardo, G.; Pozzi, S.; Pignataro, M.; Lauranzano, E.; Spano, G.; Garbelli, S.; Mantovani, S.; Marinou, K.; Papetti, L.; Monteforte, M.; et al. Amyotrophic lateral sclerosis multiprotein biomarkers in peripheral blood mononuclear cells. PLoS ONE 2011, 6, e25545. [Google Scholar] [CrossRef] [PubMed]
- Feneberg, E.; Gray, E.; Ansorge, O.; Talbot, K.; Turner, M.R. Towards a TDP-43-Based Biomarker for ALS and FTLD. Mol. Neurobiol. 2018, 55, 7789–7801. [Google Scholar] [CrossRef]
- Vanni, S.; Colini Baldeschi, A.; Zattoni, M.; Legname, G. Brain aging: A Ianus-faced player between health and neurodegeneration. J. Neurosci. Res. 2020, 98, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Devos, D.; Cabantchik, Z.I.; Moreau, C.; Danel, V.; Mahoney-Sanchez, L.; Bouchaoui, H.; Gouel, F.; Rolland, A.S.; Duce, J.A.; Devedjian, J.C. FAIRPARK-II and FAIRALS-II studygroups. Conservative iron chelation for neurodegenerative diseases such as Parkinson’s disease and amyotrophic lateral sclerosis. J. Neural Transm. 2020, 127, 189–203. [Google Scholar] [CrossRef]
- Paydarnia, P.; Mayeli, M.; Shafie, M.; Agah, E.; Hasani, S.A.; Jazani, M.R.; Sarraf, P. Alterations of the serum and CSF ferritin levels and the diagnosis and prognosis of amyotrophic lateral sclerosis. eNeurologicalSci 2021, 25, 100379. [Google Scholar] [CrossRef]
- Vanni, S.; Zattoni, M.; Moda, F.; Giaccone, G.; Tagliavini, F.; Haïk, S.; Deslys, J.P.; Zanusso, G.; Ironside, J.W.; Carmona, M.; et al. Hemoglobin mRNA Changes in the Frontal Cortex of Patients with Neurodegenerative Diseases. Front. Neurosci. 2018, 12, 8. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Cleeter, M.W.; Xuereb, J.; Taanman, J.W.; Cooper, J.M.; Schapira, A.H. Biochemical abnormalities and excitotoxicity in Huntington’s disease brain. Ann. Neurol. 1999, 45, 25–32. [Google Scholar] [CrossRef]
- Chen, C.M.; Wu, Y.R.; Chang, K.H. Altered Aconitase 2 Activity in Huntington’s Disease Peripheral Blood Cells and Mouse Model Striatum. Int. J. Mol. Sci. 2017, 18, 2480. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Baglioni, M.; Cecchetti, R.; Kivipelto, M.; Ruggiero, C.; Piobbico, D.; Kussmaul, L.; Monastero, R.; Brancorsini, S.; Mecocci, P. Lymphocytic mitochondrial aconitase activity is reduced in Alzheimer’s disease and mild cognitive impairment. J. Alzheimer’s Dis. 2015, 44, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Zweig, M.H.; Broste, S.K.; Reinhart, R.A. ROC curve analysis: An example showing the relationships among serum lipid and apolipoprotein concentrations in identifying patients with coronary artery disease. Clin. Chem. 1992, 38 Pt 1, 1425–1428. [Google Scholar] [CrossRef] [PubMed]





| ALS | Control | |
|---|---|---|
| Sex (male/female) | 15/11 | 11/11 |
| Age (years) | 58.24 ± 14.85 | 57.60 ± 11.13 |
| Riluzole | 26 | 0 |
| Survival > 36 months | 14 | - |
| Survival > 48 months | 6 | - |
| Site of onset (bulbar/spinal) | 4/22 | - |
| UMN/LMN at onset | 6/20 | - |
| VCM < 50% | 10 | - |
| BMI < 20 | 2 | - |
| ALSFRS-r High Medium Mild | 6 11 9 | - |
| El Escorial criteria Definite Probable Laboratory-supported probable | 13 10 3 | |
| Aco2 activity | 257.65 ± 111.1 mU/mg | 375.41 ± 166 mU/mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Mingot, C.; Miana-Mena, F.J.; Iñarrea, P.J.; Iñiguez, C.; Capablo, J.L.; Osta, R.; Gil-Sánchez, A.; Brieva, L.; Larrodé, P. Mitochondrial Aconitase Enzymatic Activity: A Potential Long-Term Survival Biomarker in the Blood of ALS Patients. J. Clin. Med. 2023, 12, 3560. https://doi.org/10.3390/jcm12103560
González-Mingot C, Miana-Mena FJ, Iñarrea PJ, Iñiguez C, Capablo JL, Osta R, Gil-Sánchez A, Brieva L, Larrodé P. Mitochondrial Aconitase Enzymatic Activity: A Potential Long-Term Survival Biomarker in the Blood of ALS Patients. Journal of Clinical Medicine. 2023; 12(10):3560. https://doi.org/10.3390/jcm12103560
Chicago/Turabian StyleGonzález-Mingot, Cristina, Francisco Javier Miana-Mena, Pedro José Iñarrea, Cristina Iñiguez, José Luis Capablo, Rosario Osta, Anna Gil-Sánchez, Luis Brieva, and Pilar Larrodé. 2023. "Mitochondrial Aconitase Enzymatic Activity: A Potential Long-Term Survival Biomarker in the Blood of ALS Patients" Journal of Clinical Medicine 12, no. 10: 3560. https://doi.org/10.3390/jcm12103560