Improved Monitoring of Grapholita molesta (Lepidoptera: Tortricidae) in Stone Fruit Orchards with a Pheromone-Kairomone Combination Lure
Abstract
:1. Introduction
2. Materials and Methods
3. Results
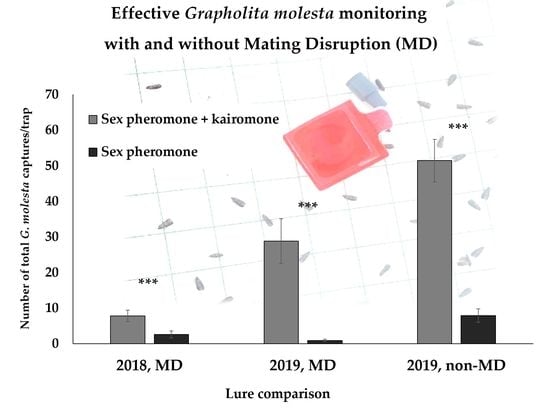
3.1. Comprehensive Analyses on G. molesta
3.2. Single-Trial Analyses
3.3. Data Analyses on Non-Target Species and Trap Color Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, X.F.; Fan, F.; Ma, C.S.; Wang, C.; Wei, G.S. Effect of host plants on the development, survivorship and reproduction of Grapholita molesta (Lepidoptera: Tortricidae) under laboratory conditions. Austral Entomol. 2016, 55, 433–438. [Google Scholar] [CrossRef]
- Cravedi, P.; Guarino, F.; Tocci, A. Valuations about mating disruption method application in Cydia molesta (Busck) control on nearly 400 hectares of peach tree in the Plane of Sibari (Calabria, South Italy). IOBC WPRS Bull. 2001, 24, 79–84. [Google Scholar]
- Sexton, S.B.; Il’ichev, A.L. Pheromone mating disruption with reference to oriental fruit moth Grapholita molesta (Busck) (Lepidoptera: Tortricidae) literature review. Gen. App. Ent. 2000, 29, 63–68. [Google Scholar] [CrossRef]
- Il’ichev, A.L.; Gut, L.J.; Williams, D.G.; Hossain, M.S.; Jerie, P.H. Area-wide approach for improved control of oriental fruit moth Grapholita molesta (Busck) (Lepidoptera: Tortricidae) by mating disruption. Gen. App. Ent. 2002, 31, 7–15. [Google Scholar]
- Figueredo, A.J.; Baker, T.C. Reduction of the response to sex pheromone in the oriental fruit moth, Grapholita molesta (Lepidoptera: Tortricidae) following successive pheromonal exposures. J. Insect Behav. 1992, 5, 347–363. [Google Scholar] [CrossRef]
- Kong, W.N.; Li, J.; Fan, R.J.; Li, S.C.; Ma, R.Y. Sex-pheromone-mediated mating disruption technology for the oriental fruit moth, Grapholita molesta (Busck) (Lepidoptera: Tortricidae): Overview and prospects. Psyche J. Entomol. 2014, 2014, 253924. [Google Scholar] [CrossRef] [Green Version]
- Maini, S.; Accinelli, G. Mating disruption-confusion method and sexual distraction: Comparison among different dispenser types for Cydia molesta (Busck) (Lepidoptera Tortricidae). Boll. Ist. Entomol. Guido Grandi Univ. Stud. Bologna 2000, 54, 113–122. [Google Scholar]
- Il’ichev, A.L.; Williams, D.G.; Drago, A. Distribution of the oriental fruit moth Grapholita molesta Busck (Lep., Tortricidae) infestation on newly planted peaches before and during 2 years of mating disruption. J. Appl. Entomol. 2003, 127, 348–353. [Google Scholar] [CrossRef]
- Preti, M.; Knight, A.L.; Angeli, S. Improving Grapholita molesta monitoring in peach and nectarine orchards under mating disruption by using bisexual lures. IOBC WPRS Bull. 2019, 146, 176–180. [Google Scholar]
- Natale, D. Olfactory Cues in Host Plant Location of the Oriental Fruit Moth, Cydia Molesta. Ph.D. Thesis, ETH Zürich (Doctoral Thesis ETH No. 15036), Zürich, Switzerland, 2003. [Google Scholar] [CrossRef]
- Piñero, J.C.; Dorn, S. Synergism between aromatic compounds and green leaf volatiles derived from the host plant underlies female attraction in the oriental fruit moth. Entomol. Exp. Appl. 2007, 125, 185–194. [Google Scholar] [CrossRef]
- Il’ichev, A.L.; Kugimiya, S.; Williams, D.G.; Takabayashi, J. Volatile compounds from young peach shoots attract males of oriental fruit moth in the field. J. Plant Interact. 2009, 4, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Varela, N.; Avilla, J.; Anton, S.; Gemeno, C. Synergism of pheromone and host-plant volatile blends in the attraction of Grapholita molesta males. Entomol. Exp. Appl. 2011, 141, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Xiang, H.M.; Chen, Z.; Li, X.W.; Guo, Y.Q.; Ma, R.Y. Two terpenoids activates close mating behavior and enhances trap efficiency of sex pheromone of Grapholita molesta. J. Asia-Pac. Entomol. 2019, 22, 1109–1114. [Google Scholar] [CrossRef]
- Cichon, L.; Fuentes-Contreras, E.; Garrido, S.; Lago, J.; Barros-Parada, W.; Basoalto, E.; Hilton, R.; Knight, A. Monitoring oriental fruit moth (Lepidoptera: Tortricidae) with sticky traps baited with terpinyl acetate and sex pheromone. J. Appl. Entomol. 2013, 137, 275–281. [Google Scholar] [CrossRef]
- Knight, A.; Basoalto, E.; Hilton, R.; Molinari, F.; Zoller, B.; Hansen, R.; Krawczyk, G.; Hull, L. Monitoring oriental fruit moth (Lepidoptera: Tortricidae) with the Ajar bait trap in orchards under mating disruption. J. Appl. Entomol. 2013, 137, 650–660. [Google Scholar] [CrossRef]
- Padilha, A.C.; Arioli, C.J.; Boff, M.I.C.; Rosa, J.M.; Botton, M. Traps and baits for luring Grapholita molesta (Busck) adults in mating disruption-treated apple orchards. Neotrop. Entomol. 2018, 47, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Knight, A.; Cichon, L.; Lago, J.; Fuentes-Contreras, E.; Barros-Parada, W.; Hull, L.; Krawczyk, G.; Zoller, B.; Hansen, R.; Hilton, R.; et al. Monitoring oriental fruit moth and codling moth (Lepidoptera: Tortricidae) with combinations of pheromones and kairomones. J. Appl. Entomol. 2014, 138, 783–794. [Google Scholar] [CrossRef]
- Knight, A.L.; Hilton, R.; Basoalto, E.; Stelinski, L.L. Use of glacial acetic acid to enhance bisexual monitoring of tortricid pests with kairomone lures in pome fruits. Environ. Entomol. 2014, 43, 1628–1640. [Google Scholar] [CrossRef] [Green Version]
- Mujica, V.; Preti, M.; Basoalto, E.; Cichon, L.; Fuentes-Contreras, E.; Barros-Prada, W.; Krawczyk, G.; Nunes, M.Z.; Walgenbach, J.F.; Hansen, R.; et al. Improved monitoring of oriental fruit moth (Lepidoptera: Tortricidae) with terpinyl acetate plus acetic acid membrane lures. J. Appl. Entomol. 2018, 142, 731–744. [Google Scholar] [CrossRef]
- Meier, U.; Graf, H.; Hack, H.; Hess, M.; Kennel, W.; Klose, R.; Mappes, D.; Seipp, D.; Stauss, R.; Streif, J.; et al. Phanologische Entwicklungsstadien des Kernobstes (Malus domestica Borkh. und Pyrus communis L.), des Steinobstes (Prunus-Arten), der Johannisbeere Ribes-Arten) und der Erdbeere (Fragaria x ananassa Duch.). Nachr. Dtsch. Pflanzenschutzd. 1994, 199446, 141–153. [Google Scholar]
- Plantgest. 2020. Available online: https://plantgest.imagelinenetwork.com/it/ (accessed on 4 June 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 4 June 2020).
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Riedl, H.; Croft, B.A. A study of pheromone trap catches in relation to codling moth (Lepidoptera: Olethreutidae) damage. Can. Entomol. 1974, 106, 525–537. [Google Scholar] [CrossRef]
- Clare, G.; Suckling, D.M.; Bradley, S.J.; Walker, J.T.S.; Shaw, P.W.; Daly, J.M.; Mclaren, G.F.; Wearing, C.H. Pheromone trap colour determines catch of nontarget insects. N. Z. Plant Prot. 2000, 53, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Myers, C.T.; Krawczyk, G.; Agnello, A.M. Response of tortricid moths and non-target insects to pheromone trap color in commercial apple orchards. J. Entomol. Sci. 2009, 44, 69–77. [Google Scholar] [CrossRef]
- Knight, A.L. Multiple mating of male and female codling moth (Lepidoptera: Tortricidae) in apple orchards treated with sex pheromone. Environ. Entomol. 2014, 36, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, B.D.; Guédot, C.; Landolt, P.J. Mass-trapping codling moth, Cydia pomonella (Lepidoptera: Torticidae), using a kairomone lure reduces fruit damage in commercial apple orchards. J. Econ. Entomol. 2018, 111, 1983–1986. [Google Scholar] [CrossRef]
- Stelinski, L.; Holdcraft, R.; Rodriguez-Saona, C. Female moth calling and flight behavior are altered hours following pheromone autodetection: Possible implications for practical management with mating disruption. Insects 2014, 5, 459–473. [Google Scholar] [CrossRef] [Green Version]
- Yetter, W.P.; Steiner, L.F. A preliminary report on large-scale bait trapping of the oriental fruit moth in Indiana and Georgia. J. Econ. Entomol. 1931, 24, 1181–1197. [Google Scholar] [CrossRef]
- Kovanci, O.B.; Walgenbach, J.F. Monitoring the oriental fruit moth with pheromone and bait traps in apple orchards under different management regimes. Int. J. Pest Manag. 2005, 51, 273–279. [Google Scholar] [CrossRef]
- Roelofs, W.; Comeau, A.; Selle, R. Sex pheromone of the oriental fruit moth. Nature 1969, 224, 723. [Google Scholar] [CrossRef]
- Phillips, J.H.H. Monitoring for oriental fruit moth with synthetic sex pheromone. Environ. Entomol. 1973, 2, 1039–1042. [Google Scholar] [CrossRef]
- El-Shafie, H.A.F.; Faleiro, J.R. Semiochemicals and their potential use in pest management. In Biological Control of Pest and Vector Insects; Shields, V., Ed.; InTechOpen: London, UK, 2017; pp. 1–10. [Google Scholar] [CrossRef] [Green Version]
- Maini, S.; Burgio, G. Ostrinia nubilalis (Hb.) (Lep., Pyralidae) on sweet corn: Relationship between adults caught in multibaited traps and ear damages. J. Appl. Entomol. 1999, 123, 179–185. [Google Scholar] [CrossRef]
- Najar-Rodriguez, A.; Orschel, B.; Dorn, S. Season-long volatile emissions from peach and pear trees in situ, overlapping profiles, and olfactory attraction of an oligophagous fruit moth in the laboratory. J. Chem. Ecol. 2013, 39, 418–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.F.; Huang, L.Q.; Wang, C.Z. Identification and field evaluation of pear fruit volatiles attractive to the oriental fruit moth, Cydia molesta. J. Chem. Ecol. 2012, 38, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.F.; Qiao, H.L.; Xu, Z.C.; Cheng, J.; Zong, S.X.; Luo, Y.Q. Comparative analysis of peach and pear fruit volatiles attractive to the oriental fruit moth, Cydia molesta. J. Plant. Interact. 2014, 9, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.F.; Wang, R.; Wang, C.Z.; Luo, Y.Q.; Qiao, H.L. Sexual differences in electrophysiological and behavioral responses of Cydia molesta to peach and pear volatiles. Entomol. Exp. Appl. 2015, 157, 279–290. [Google Scholar] [CrossRef]
- Barros-Parada, W.; Ammagarahalli, B.; Basoalto, E.; Fuentes-Contreras, E.; Gemeno, C. Captures of oriental fruit moth, Grapholita molesta (Lepidoptera: Tortricidae), in traps baited with host-plant volatiles in Chile. Appl. Entomol. Zool. 2018, 53, 193–204. [Google Scholar] [CrossRef]

| Trial # | Site Management | Peach or Nectarine Cultivar (Estimated Harvest Date) | Trial Start Date | Crop Status During Trial | Trap Color | N° Traps per Trt. |
|---|---|---|---|---|---|---|
| 1 | MD | Romagna Red (July 3) | 24 April 2018 | Early fruit devel. | Orange | 5 |
| 2 | MD | Romagna 3000 (August 30) | 24 April 2018 | Early fruit devel. | Orange | 5 |
| 3 | MD | Romagna Big (July 19) | 5 May 2018 | Late fruit devel. | Orange | 5 |
| 4 | MD | Max 7 (September 5) | 1 June 2018 | Early fruit devel. | Orange | 5 |
| 5 | MD | August Red (August 31) | 11 June 2018 | Late fruit devel. | White | 6 |
| 6 | MD | Fercluse (July 15) | 8 June 2018 | Late fruit devel. | White | 6 |
| 7 | MD | Lami Puntoit (August 15) | 8 June 2018 | Late fruit devel. | White | 6 |
| 8 | MD | Red Late (September 1) | 25 July 2018 | Late fruit devel. | White | 5 |
| 9 | MD | Sweet Lady (August 17) | 26 July 2018 | Late fruit devel. | White | 5 |
| 10 | MD | Tardibelle (September 18) | 1 June 2018 | Early fruit devel. | Orange | 5 |
| 11 | MD | August Red (August 31) | 1 June 2018 | Late fruit devel. | White | 5 |
| 12 | MD | Romagna 3000 (August 30) | 1 June 2018 | Late fruit devel. | White | 5 |
| 13 | MD | Fairlane (September 5) | 1 June 2018 | Early fruit devel. | Orange | 5 |
| 14 | MD | Romagna 3000 (August 30) | 8 March 2019 | Early fruit devel. | White | 5 |
| 15 | MD | Romagna Big (July 19) | 8 March 2019 | Early fruit devel. | White | 5 |
| 16 | MD | Early Fresh (June 5) | 8 March 2019 | Early fruit devel. | White | 5 |
| 17 | Non-MD | Big Top (July 7) | 8 March 2019 | Early fruit devel. | White | 5 |
| 18 | Non-MD | Diamond Ray (July 22) | 10 May 2019 | Late fruit devel. | White | 5 |
| 19 | Non-MD | Ibla (August 16) | 10 May 2019 | Early fruit devel. | White | 5 |
| 20 | MD | Romagna 3000 (August 30) | 10 May 2019 | Late fruit devel. | White | 5 |
| 21 | MD | Romagna Big (July 19) | 10 May 2019 | Late fruit devel. | White | 5 |
| 22 | MD | Romagna Red (July 3) | 10 May 2019 | Late fruit devel. | White | 5 |
| 23 | MD | Early Fresh (June 5) | 21 May 2019 | Late fruit devel. | White | 5 |
| 24 | Non-MD | Big Top (July 7) | 10 May 2019 | Late fruit devel. | White | 5 |
| 25 | Non-MD | Diamond Ray (July 22) | 15 July 2019 | Post-Harvest | White | 5 |
| 26 | MD | Early Fresh (June 5) | 15 July 2019 | Post-Harvest | White | 5 |
| 27 | Non-MD | Big Top (July 7) | 15 July 2019 | Post-Harvest | White | 5 |
| 28 | MD | Romagna 3000 (August 30) | 15 July 2019 | Late fruit devel. | White | 5 |
| 29 | MD | Romagna Big (July 19) | 15 July 2019 | Post-Harvest | White | 5 |
| 30 | MD | Romagna Red (July 3) | 15 July 2019 | Post-Harvest | White | 5 |
| 31 | Non-MD | Diamond Ray (July 22) | 27 August 2019 | Post-Harvest | White | 5 |
| 32 | Non-MD | Early Fresh (June 5) | 27 August 2019 | Post-Harvest | White | 5 |
| 33 | Non-MD | Big Top (July 7) | 26 August 2019 | Post-Harvest | White | 5 |
| Trial #(a) | Cultivar (Peach or Nectarine) | Orchard Size (ha) | Geographical Coordinates (Latitude Longitude) | MD Type and Density Applied (Dispensers ha−1 or Dosage ha−1) |
|---|---|---|---|---|
| 1, 22, 30 | Romagna Red (nectarine) | 1.5 | 44°19’14.53”N-11°51’35.67”E | ISOMATE® OFM rosso FLEX at 740 |
| 2, 12, 14, 20, 28 | Romagna 3000 (nectarine) | 1.5 | 44°19’6.62”N-11°51’33.63”E | ISOMATE® OFM rosso FLEX at 740 |
| 3, 15, 21, 29 | Romagna Big (nectarine) | 1.5 | 44°19’11.24”N-11°51’47.47”E | ISOMATE® OFM rosso FLEX at 740 |
| 4 | Max 7 (nectarine) | 1.5 | 44°19’22.52”N-11°51’32.87”E | ISOMATE® OFM rosso FLEX at 700 |
| 5 | August Red (nectarine) | 1.8 | 44°16’50.88”N-12°3’37.77”E | CheckMate OFM®-F (spray) at 100 ml |
| 6 | Fercluse (peach) | 6.0 | 44°28’36.45”N-12°5’4.18”E | ISOMATE® OFM rosso FLEX at 650 |
| 7 | Lami Puntoit (peach) | 5.0 | 44°28’41.19”N-12°5’7.40”E | ISOMATE® OFM rosso FLEX at 650 |
| 8 | Red Late (nectarine) | 7.0 | 44°16’24.99”N-12°2’30.74”E | ISOMATE® OFM rosso FLEX at 700 |
| 9 | Sweet Lady (nectarine) | 1.5 | 44°23’52.61”N-12°0’54.12”E | RAK® 5+6 at 700 |
| 10 | Tardibelle (peach) | 4.5 | 44°16’46.63”N-12°2’41.86”E | Check Mate Puffer OFM at 3.5 |
| 11 | August Red (nectarine) | 2.8 | 44°17’0.08”N-12°3’24.56”E | CheckMate® OFM at 350 |
| 13 | Fairlane (nectarine) | 2.3 | 44°19’40.07”N-11°52’28.81”E | RAK® 5+6 at 650 |
| 16, 23, 26, 32 | Early Fresh (peach) | 5.4 | 44°19’41.32”N-11°52’25.25”E | CheckMate OFM®-F (spray) at 50 ml |
| 17, 24, 27, 33 | Big Top (nectarine) | 1.5 | 44°15’50.29”N-11°53’10.73”E | Untreated with MD |
| 18, 25, 31 | Diamond Ray (nectarine) | 1.5 | 44°19’28.13”N-11°51’12.16”E | Untreated with MD |
| 19 | Ibla (peach) | 2.0 | 44°19’36.70”N-11°51’7.65”E | Untreated with MD |
| Year, Management | Mean (± SEM) G. molesta Captures | Lure | Statistics (glmmTMB Outputs) | |
|---|---|---|---|---|
| Combo Dual | Pheromone | |||
| 2018, MD | Males | 7.1 ± 1.5 | 2.6 ± 1.0 | df = 124, z = −5.687, p < 0.001 |
| Total | 7.8 ± 1.6 | 2.6 ± 1.0 | df = 124, z = −6.411, p < 0.001 | |
| Proportion of females | 0.08 | 0.00 | - | |
| 2019, MD | Males | 27.6 ± 6.3 | 0.9 ± 0.3 | df = 97, z = −20.119, p < 0.001 |
| Total | 28.8 ± 6.3 | 0.9 ± 0.3 | df = 97, z = −19.923, p < 0.001 | |
| Proportion of females | 0.04 | 0.00 | - | |
| 2019, non-MD | Males | 46.7 ± 5.3 | 7.9 ± 1.9 | df = 77, z = −14.277, p < 0.001 |
| Total | 51.4 ± 6.0 | 7.9 ± 1.9 | df = 77, z = −15.069, p < 0.001 | |
| Proportion of females | 0.09 | 0.00 | - | |
| Explanatory Variable | Dataset Analyzed | G. molesta Captures | Statistics (glmmTMB Outputs) |
|---|---|---|---|
| Year (2018 vs 2019) | 2018 MD and 2019 MD | Males | df = 232, z = 0.667, p = 0.505 |
| Total | df = 232, z = 0.736, p = 0.462 | ||
| Management (MD vs non-MD) | 2019 (MD and non-MD) | Males | df = 191, z = 2.083, p = 0.037 |
| Total | df = 191, z = 2.002, p = 0.045 | ||
| Crop type (peach vs nectarine) | 2018 MD | Males | df = 124, z = −2.250, p = 0.025 |
| Total | df = 124, z = −2.200, p = 0.028 | ||
| Crop phenology (early vs late) | 2018 MD | Males | df = 124, z = −0.372, p = 0.710 |
| Total | df = 124, z = −0.400, p = 0.690 | ||
| Crop type (peach vs nectarine) | 2019 MD | Males | df = 97, z = 1.916, p = 0.055 |
| Total | df = 97, z = 1.733, p = 0.083 | ||
| Crop phenology (early vs late) | 2019 MD | Males | df = 97, z = −3.965, p < 0.001 |
| Total | df = 97, z = −4.746, p < 0.001 | ||
| Crop phenology (early vs post-harvest) | 2019 MD | Males | df = 97, z = −1.602, p < 0.109 |
| Total | df = 97, z = −2.234, p < 0.026 | ||
| Crop type (peach vs nectarine) | 2019 non-MD | Males | df = 77, z = −1.621, p = 0.105 |
| Total | df = 77, z = −1.588, p = 0.112 | ||
| Crop phenology (early vs late) | 2019 non-MD | Males | df = 77, z = 1.258, p = 0.208 |
| Total | df = 77, z = 1.248, p = 0.212 | ||
| Crop phenology (early vs post-harvest) | 2019 non-MD | Males | df = 77, z = 5.800, p < 0.001 |
| Total | df = 77, z = 5.553, p < 0.001 |
| Management | Year | Trial # | Lure Type | G. molesta Captures | Unpaired Two-Sample Test on Male Captures | |
|---|---|---|---|---|---|---|
| Females | Males | |||||
| MD | 2018 | 1 | Combo Dual | 0.8 ± 0.5 | 3.2 ± 0.7 | t = 2.810, df = 8, p = 0.023 |
| Pheromone | 0.0 ± 0.0 | 0.8 ± 0.5 | ||||
| 2 | Combo Dual | 4.6 ± 1.1 | 31.6 ± 9.9 | t = 1.140, df = 8, p = 0.287 | ||
| Pheromone | 0.0 ± 0.0 | 18.8 ± 10.9 | ||||
| 4 | Combo Dual | 0.2 ± 0.2 | 3.4 ± 0.9 | - | ||
| Pheromone | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||
| 10 | Combo Dual | 0.4 ± 0.2 | 1.4 ± 0.9 | W = 16, p = 0.441 | ||
| Pheromone | 0.0 ± 0.0 | 0.4 ± 0.4 | ||||
| 13 | Combo Dual | 0.4 ± 0.2 | 0.2 ± 0.2 | - | ||
| Pheromone | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||
| 2019 | 14 | Combo Dual | 1.2 ± 0.4 | 89.2 ± 16.3 | t = 8.647, df = 8, p < 0.001 | |
| Pheromone | 0.0 ± 0.0 | 2.0 ± 0.6 | ||||
| 15 | Combo Dual | 1.2 ± 0.5 | 11.6 ± 1.1 | - | ||
| Pheromone | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||
| 16 | Combo Dual | 3.0 ± 0.5 | 147.0 ± 4.1 | W = 25, p = 0.012 | ||
| Pheromone | 0.0 ± 0.0 | 6.6 ± 2.0 | ||||
| non-MD | 2019 | 17 | Combo Dual | 0.6 ± 0.4 | 21.6 ± 3.7 | W = 25, p = 0.010 |
| Pheromone | 0.0 ± 0.0 | 2.8 ± 0.2 | ||||
| 19 | Combo Dual | 0.0 ± 0.0 | 8.0 ± 2.0 | - | ||
| Pheromone | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||
| Management | Year | Trial # | Lure Type | G. molesta Captures | Unpaired Two-Sample Test on Male Captures | |
|---|---|---|---|---|---|---|
| Females | Males | |||||
| MD | 2018 | 3 | Combo Dual | 0.2 ± 0.2 | 3.2 ± 1.4 | t = 1.201, df = 8, p = 0.264 |
| Pheromone | 0.0 ± 0.0 | 1.2 ± 0.6 | ||||
| 5 | Combo Dual | 0.5 ± 0.3 | 7.5 ± 2.4 | t = 3.114, df = 10, p = 0.011 | ||
| Pheromone | 0.0 ± 0.0 | 1.5 ± 1.1 | ||||
| 6 | Combo Dual | 0.0 ± 0.0 | 0.3 ± 0.2 | - | ||
| Pheromone | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||
| 7 | Combo Dual | 0.3 ± 0.2 | 0.0 ± 0.0 | - | ||
| Pheromone | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||
| 8 | Combo Dual | 0.2 ± 0.2 | 0.4 ± 0.2 | - | ||
| Pheromone | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||
| 9 | Combo Dual | 1.0 ± 0.5 | 17.2 ± 4.6 | t = 1.927, df = 8, p = 0.090 | ||
| Pheromone | 0.0 ± 0.0 | 7.2 ± 2.3 | ||||
| 11 | Combo Dual | 0.0 ± 0.0 | 26.0 ± 6.3 | t = 3.193, df = 8, p = 0.013 | ||
| Pheromone | 0.0 ± 0.0 | 5.4 ± 4.9 | ||||
| 12 | Combo Dual | 0.0 ± 0.0 | 1.0 ± 0.5 | W = 18, p = 0.232 | ||
| Pheromone | 0.0 ± 0.0 | 0.2 ± 0.2 | ||||
| MD | 2019 | 20 | Combo Dual | 0.2 ± 0.2 | 11.0 ± 1.3 | W = 25, p = 0.009 |
| Pheromone | 0.0 ± 0.0 | 0.6 ± 0.6 | ||||
| 21 | Combo Dual | 0.6 ± 0.4 | 4.2 ± 1.1 | t = 5.014, df = 8, p = 0.001 | ||
| Pheromone | 0.0 ± 0.0 | 0.2 ± 0.2 | ||||
| 22 | Combo Dual | 0.2 ± 0.2 | 2.8 ± 0.4 | - | ||
| Pheromone | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||
| 23 | Combo Dual | 0.4 ± 0.2 | 3.6 ± 0.2 | - | ||
| Pheromone | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||
| 28 | Combo Dual | 1.4 ± 0.7 | 1.8 ± 0.6 | - | ||
| Pheromone | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||
| non-MD | 2019 | 18 | Combo Dual | 0.0 ± 0.0 | 7.4 ± 1.8 | t = 6.358, df = 8, p < 0.001 |
| Pheromone | 0.0 ± 0.0 | 0.2 ± 0.2 | ||||
| 24 | Combo Dual | 7.2 ± 2.4 | 43.6 ± 7.4 | t = 5.627, df = 8, p < 0.001 | ||
| Pheromone | 0.0 ± 0.0 | 7.2 ± 2.4 | ||||
| Management | Year | Trial # | Lure Type | G. molesta Captures | Unpaired Two-Sample Test on Male Captures | |
|---|---|---|---|---|---|---|
| Females | Males | |||||
| MD | 2019 | 26 | Combo Dual | 1.2 ± 0.6 | 21.2 ± 3.2 | t = 9.678, df = 8, p < 0.001 |
| Pheromone | 0.0 ± 0.0 | 0.4 ± 0.2 | ||||
| 29 | Combo Dual | 3.2 ± 1.5 | 5.8 ± 1.8 | - | ||
| Pheromone | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||
| 30 | Combo Dual | 0.8 ± 0.4 | 5.6 ± 1.7 | - | ||
| Pheromone | 0.0 ± 0.0 | 0.0 ± 0.0 | ||||
| non-MD | 2019 | 25 | Combo Dual | 3.0 ± 1.3 | 52.4 ± 4.3 | W = 25, p = 0.012 |
| Pheromone | 0.0 ± 0.0 | 0.8 ± 0.4 | ||||
| 27 | Combo Dual | 17.6 ± 1.7 | 99.8 ± 5.0 | t = 9.042, df = 8, p < 0.001 | ||
| Pheromone | 0.0 ± 0.0 | 32.8 ± 4.7 | ||||
| 31 | Combo Dual | 6.6 ± 1.9 | 80.2 ± 16.3 | t = 7.499, df = 8, p < 0.001 | ||
| Pheromone | 0.0 ± 0.0 | 2.8 ± 0.9 | ||||
| 32 | Combo Dual | 2.4 ± 0.7 | 25.4 ± 4.4 | t = 7.340, df = 8, p < 0.001 | ||
| Pheromone | 0.0 ± 0.0 | 1.2 ± 0.5 | ||||
| 33 | Combo Dual | 5.0 ± 1.9 | 81.6 ± 9.1 | t = 5.485, df = 8, p = 0.001 | ||
| Pheromone | 0.0 ± 0.0 | 23.4 ± 6.0 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preti, M.; Knight, A.L.; Angeli, S. Improved Monitoring of Grapholita molesta (Lepidoptera: Tortricidae) in Stone Fruit Orchards with a Pheromone-Kairomone Combination Lure. Insects 2020, 11, 412. https://doi.org/10.3390/insects11070412
Preti M, Knight AL, Angeli S. Improved Monitoring of Grapholita molesta (Lepidoptera: Tortricidae) in Stone Fruit Orchards with a Pheromone-Kairomone Combination Lure. Insects. 2020; 11(7):412. https://doi.org/10.3390/insects11070412
Chicago/Turabian StylePreti, Michele, Alan L. Knight, and Sergio Angeli. 2020. "Improved Monitoring of Grapholita molesta (Lepidoptera: Tortricidae) in Stone Fruit Orchards with a Pheromone-Kairomone Combination Lure" Insects 11, no. 7: 412. https://doi.org/10.3390/insects11070412