Age-Related Changes in Clinical and Analytical Variables in Chronic Hemodialyzed Patients
Abstract
:1. Introduction
2. Results
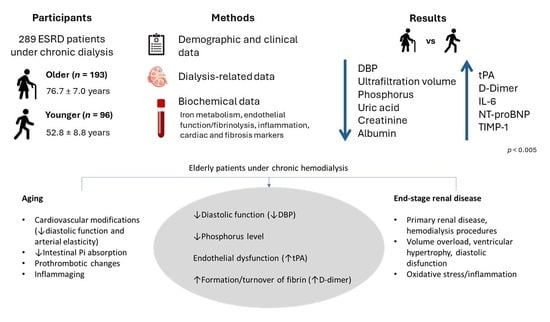
2.1. Demographic and Clinical Data of Patients
2.2. Hematological and Biochemical Data of Patients
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Erythropoiesis Stimulating Agents (ESA) and Iron Therapies
4.3. Assays
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schoolwerth, A.C.; Engelgau, M.M.; Hostetter, T.H.; Rufo, K.H.; Chianchiano, D.; McClellan, W.M.; Warnock, D.G.; Vinicor, F. Chronic kidney disease: A public health problem that needs a public health action plan. Prev. Chronic Dis. 2006, 3, A57. [Google Scholar]
- USRDS. 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2018.
- Macário, F.; Filipe, R.; Carvalho, M.J.; Galvão, M.; Lopes, J.A.; Amoedo, M.; Silva, G. Portuguese Registry of Dialysis and Transplantation 2017; Encontro Renal: Algarve, Portugal, 2018. [Google Scholar]
- Hirakawa, Y.; Jao, T.M.; Inagi, R. Pathophysiology and therapeutics of premature ageing in chronic kidney disease, with a focus on glycative stress. Clin. Exp. Pharmacol. Physiol. 2017, 44 (Suppl. S1), 70–77. [Google Scholar] [CrossRef]
- Turin, T.C.; Tonelli, M.; Manns, B.J.; Ravani, P.; Ahmed, S.B.; Hemmelgarn, B.R. Chronic kidney disease and life expectancy. Nephrol. Dial. Transplant. 2012, 27, 3182–3186. [Google Scholar] [CrossRef]
- Barja, G. Towards a unified mechanistic theory of aging. Exp. Gerontol. 2019, 124, 110627. [Google Scholar] [CrossRef]
- Khan, S.S.; Singer, B.D.; Vaughan, D.E. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell 2017, 16, 624–633. [Google Scholar] [CrossRef]
- Stauder, R.; Valent, P.; Theurl, I. Anemia at older age: Etiologies, clinical implications, and management. Blood 2018, 131, 505–514. [Google Scholar] [CrossRef]
- De Pooter, N.; Brionne-François, M.; Smahi, M.; Abecassis, L.; Toulon, P. Age-adjusted D-dimer cut-off levels to rule out venous thromboembolism in patients with non-high pre-test probability: Clinical performance and cost-effectiveness analysis. J. Thromb. Haemost. 2021, 19, 1271–1282. [Google Scholar] [CrossRef]
- Reichel, H.; Zee, J.; Tu, C.; Young, E.; Pisoni, R.L.; Stengel, B.; Duttlinger, J.; Lonnemann, G.; Robinson, B.M.; Pecoits-Filho, R.; et al. Chronic kidney disease progression and mortality risk profiles in Germany: Results from the Chronic Kidney Disease Outcomes and Practice Patterns Study. Nephrol. Dial. Transplant. 2020, 35, 803–810. [Google Scholar] [CrossRef]
- Tufan, F.; Yıldız, A.; Dogan, I.; Yıldız, D.; Sevinir, Ş. Urea to creatinine ratio: A forgotten marker of poor nutritional state in patients undergoing hemodialysis treatment. Aging Male 2015, 18, 49–53. [Google Scholar] [CrossRef]
- Snaedal, S.; Qureshi, A.R.; Lund, S.H.; Germanis, G.; Hylander, B.; Heimbürger, O.; Carrero, J.J.; Stenvinkel, P.; Bárány, P. Dialysis modality and nutritional status are associated with variability of inflammatory markers. Nephrol. Dial. Transplant. 2016, 31, 1320–1327. [Google Scholar] [CrossRef]
- Crimmins, E.; Vasunilashorn, S.; Kim, J.K.; Alley, D. Biomarkers related to aging in human populations. Adv. Clin. Chem. 2008, 46, 161–216. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E. Blood pressure and ageing. Postgrad. Med. J. 2007, 83, 109–114. [Google Scholar] [CrossRef]
- Tschöpe, C.; Kasner, M.; Westermann, D.; Gaub, R.; Poller, W.C.; Schultheiss, H.P. The role of NT-proBNP in the diagnostics of isolated diastolic dysfunction: Correlation with echocardiographic and invasive measurements. Eur. Heart J. 2005, 26, 2277–2284. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Xiao, W.; Liu, Y.; Wu, H.; Ye, P.; Sheng, L. NT-proBNP is associated with age, gender and glomerular filtration rate in a community-dwelling population. Int. J. Clin. Exp. Med. 2019, 12, 12220–12227. [Google Scholar]
- Franklin, S.S.; Larson, M.G.; Khan, S.A.; Wong, N.D.; Leip, E.P.; Kannel, W.B.; Levy, D. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001, 103, 1245–1249. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef]
- Mari, D.; Ogliari, G.; Castaldi, D.; Vitale, G.; Bollini, E.M.; Lio, D. Hemostasis and ageing. Immun. Ageing 2008, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M. Hemostasis and aging. Crit. Rev. Oncol. Hematol. 2006, 60, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, M.; Evrin, P.E.; Lundblad, D.; Asplund, K.; Rånby, M. Influence of gender, age and sampling time on plasma fibrinolytic variables and fibrinogen: A population study. Fibrinolysis 1993, 7, 316–323. [Google Scholar] [CrossRef]
- Smith, D.T.; Hoetzer, G.L.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Effects of ageing and regular aerobic exercise on endothelial fibrinolytic capacity in humans. J. Physiol. 2003, 546, 289–298. [Google Scholar] [CrossRef]
- do Sameiro Faria, M.; Ribeiro, S.; Nascimento, H.; Rocha-Pereira, P.; Miranda, V.; Mendonça, D.; Quintanilha, A.; Costa, E.; Belo, L.; Santos-Silva, A. Adiponectin is an independent predictor of tissue plasminogen activator levels in patients under haemodialysis. Scand. J. Urol. Nephrol. 2012, 46, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Tita-Nwa, F.; Bos, A.; Adjei, A.; Ershler, W.B.; Longo, D.L.; Ferrucci, L. Correlates of D-dimer in older persons. Aging Clin. Exp. Res. 2010, 22, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Lertdumrongluk, P.; Rhee, C.M.; Park, J.; Lau, W.L.; Moradi, H.; Jing, J.; Molnar, M.Z.; Brunelli, S.M.; Nissenson, A.R.; Kovesdy, C.P.; et al. Association of serum phosphorus concentration with mortality in elderly and nonelderly hemodialysis patients. J. Ren. Nutr. 2013, 23, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Scanni, R.; vonRotz, M.; Jehle, S.; Hulter, H.N.; Krapf, R. The human response to acute enteral and parenteral phosphate loads. J. Am. Soc. Nephrol. 2014, 25, 2730–2739. [Google Scholar] [CrossRef] [PubMed]
- Mulroney, S.E.; Woda, C.; Haramati, A. Changes in renal phosphate reabsorption in the aged rat. Proc. Soc. Exp. Biol. Med. 1998, 218, 62–67. [Google Scholar] [CrossRef]
- Lorenzo, V.; Martín, M.; Rufino, M.; Jiménez, A.; Malo, A.M.; Sanchez, E.; Hernández, D.; Rodríguez, M.; Torres, A. Protein intake, control of serum phosphorus, and relatively low levels of parathyroid hormone in elderly hemodialysis patients. Am. J. Kidney Dis. 2001, 37, 1260–1266. [Google Scholar] [CrossRef]
- Vorland, C.J.; Lachcik, P.J.; Aromeh, L.O.; Moe, S.M.; Chen, N.X.; Hill Gallant, K.M. Effect of dietary phosphorus intake and age on intestinal phosphorus absorption efficiency and phosphorus balance in male rats. PLoS ONE 2018, 13, e0207601. [Google Scholar] [CrossRef]
- Post, A.; Tsikas, D.; Bakker, S.J.L. Creatine is a Conditionally Essential Nutrient in Chronic Kidney Disease: A Hypothesis and Narrative Literature Review. Nutrients 2019, 11, 1044. [Google Scholar] [CrossRef]
- Hwang, W.; Cho, M.S.; Oh, J.E.; Lee, J.H.; Jeong, J.C.; Shin, G.T.; Kim, H.; Park, I. Comparison of creatinine index and geriatric nutritional risk index for nutritional evaluation of patients with hemodialysis. Hemodial. Int. 2018, 22, 507–514. [Google Scholar] [CrossRef]
- Park, J.; Mehrotra, R.; Rhee, C.M.; Molnar, M.Z.; Lukowsky, L.R.; Patel, S.S.; Nissenson, A.R.; Kopple, J.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum creatinine level, a surrogate of muscle mass, predicts mortality in peritoneal dialysis patients. Nephrol. Dial. Transplant. 2013, 28, 2146–2155. [Google Scholar] [CrossRef]
- Bouillanne, O.; Golmard, J.L.; Coussieu, C.; Noël, M.; Durand, D.; Piette, F.; Nivet-Antoine, V. Leptin a new biological marker for evaluating malnutrition in elderly patients. Eur. J. Clin. Nutr. 2007, 61, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.E.; Sullivan, S.; Harten, I.; Schneider, S.H.; Greenberg, A.S.; Fried, S.K. Interleukin-6 regulates human adipose tissue lipid metabolism and leptin production in vitro. J. Clin. Endocrinol. Metab. 2004, 89, 5577–5582. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.J.; Rocha, S.; Coimbra, S.; Catarino, C.; Rocha-Pereira, P.; Bronze-da-Rocha, E.; Oliveira, J.G.; Madureira, J.; Fernandes, J.C.; do Sameiro-Faria, M.; et al. Long Pentraxin 3 as a Broader Biomarker for Multiple Risk Factors in End-Stage Renal Disease: Association with All-Cause Mortality. Mediat. Inflamm. 2019, 2019, 3295725. [Google Scholar] [CrossRef] [PubMed]
- Meuwese, C.L.; Stenvinkel, P.; Dekker, F.W.; Carrero, J.J. Monitoring of inflammation in patients on dialysis: Forewarned is forearmed. Nat. Rev. Nephrol. 2011, 7, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Nilsson-Ehle, H.; Jagenburg, R.; Landahl, S.; Svanborg, A. Blood haemoglobin declines in the elderly: Implications for reference intervals from age 70 to 88. Eur. J. Haematol. 2000, 65, 297–305. [Google Scholar] [CrossRef]
- Belo, L.; Rocha, S.; Valente, M.J.; Coimbra, S.; Catarino, C.; Bronze-da-Rocha, E.; Rocha-Pereira, P.; do Sameiro-Faria, M.; Oliveira, J.G.; Madureira, J.; et al. Hepcidin and diabetes are independently related with soluble transferrin receptor levels in chronic dialysis patients. Ren. Fail. 2019, 41, 662–672. [Google Scholar] [CrossRef]
- Huebers, H.A.; Beguin, Y.; Pootrakul, P.; Einspahr, D.; Finch, C.A. Intact transferrin receptors in human plasma and their relation to erythropoiesis. Blood 1990, 75, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J.; Skikne, B.S.; Simpson, K.M.; Baynes, R.D.; Cook, J.D. Serum transferrin receptor distinguishes the anemia of chronic disease from iron deficiency anemia. J. Lab. Clin. Med. 1992, 119, 385–390. [Google Scholar] [PubMed]
- Ashby, D.; Busbridge, M.; Hildebrand, S.; Clarke, C.; Aldous, G.; Palan, M.; Murphy, K.; Duncan, N.; Choi, P. Hepcidin clearance is associated with erythropoietin requirement in stable hemodialysis patients. Clin. Nephrol. 2017, 87, 231–236. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Hong, Q.; Lin, H.; Zhu, H.; Liu, Q.; Wang, J.; Xie, Y.; Shang, X.; Shi, S.; et al. TIMP-1 promotes age-related renal fibrosis through upregulating ICAM-1 in human TIMP-1 transgenic mice. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1130–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Rakheja, D.; Yu, X.; Saxena, R.; Vaziri, N.D.; Silva, F.G. The aging kidney. Kidney Int. 2008, 74, 710–720. [Google Scholar] [CrossRef] [PubMed]
- ADA. Standards of Medical Care in Diabetes—2017. Diabetes Care 2017, 40, S1–S135. [Google Scholar]
- Locatelli, F.; Bárány, P.; Covic, A.; De Francisco, A.; Del Vecchio, L.; Goldsmith, D.; Hörl, W.; London, G.; Vanholder, R.; Van Biesen, W. Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: A European Renal Best Practice position statement. Nephrol. Dial. Transplant. 2013, 28, 1346–1359. [Google Scholar] [CrossRef]
- WHOCC. ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 28 January 2021).
- Daugirdas, J.T. Simplified equations for monitoring Kt/V, PCRn, eKt/V, and ePCRn. Adv. Ren. Replace. Ther. 1995, 2, 295–304. [Google Scholar] [CrossRef]



| Total (n = 289) | Younger (n = 96) | Older (n = 193) | p | |
|---|---|---|---|---|
| Age, years | 68.7 ± 13.6 | 52.8 ± 8.8 | 76.7 ± 7.0 | |
| Gender, n (%) | ||||
| Male | 158 (54.7) | 55 (57.3) | 103 (53.4) | 0.534 |
| Female | 131 (45.3) | 41 (42.7) | 90 (46.6) | |
| BMI, Kg/m2 | 25.8 ± 4.6 | 25.3 ± 4.8 | 25.9 ± 4.4 | 0.282 |
| Systolic blood pressure (mm Hg) | 137.7 ± 21.0 | 138.1 ± 23.9 | 137.5 ± 19.5 | 0.831 |
| Diastolic blood pressure (mm Hg) | 63.1 ± 12.8 | 71.6 ± 12.2 | 58.8 ± 10.9 | <0.001 |
| Etiology of CKD, n (%) | ||||
| Diabetic nephropathy | 101 (34.9) | 26 (27.1) | 75 (38.9) | <0.001 * |
| Hypertensive nephrosclerosis | 36 (12.5) | 11 (11.5) | 25 (13.0) | |
| Polycystic kidney disease | 19 (6.6) | 9 (9.4) | 10 (5.2) | |
| Chronic glomerulonephritis | 23 (8.0) | 15 (15.6) | 8 (4.1) | |
| Other or undetermined | 110 (38.0) | 35 (36.4) | 75 (38.8) | |
| Dialysis Vintage, Years | 3.74 (1.65–7.40) | 3.45 (1.70–7.01) | 3.79 (1.61–7.51) | 0.967 |
| Dialysis Therapy, n (%) | ||||
| Hemodialysis | 41 (14.2) | 10 (10.4) | 31 (16.1) | 0.215 * |
| On-line hemodiafiltration | 248 (85.8) | 162 (83.9) | 86 (89.6) | |
| Vascular Access, n (%) | ||||
| Arteriovenous fistula | 233 (80.6) | 72 (75.0) | 161 (83.4) | 0.181 * |
| Arteriovenous graft | 14 (4.8) | 7 (7.3) | 7 (3.6) | |
| Central venous catheter | 42 (14.5) | 17 (17.7) | 25 (13.0) | |
| Intradialytic therapy | ||||
| ESA prescription, n (%) | 240 (83.0) | 78 (81.3) | 162 (83.9) | 0.618 |
| ESA dose (µg/Kg/week) | 0.36 (0.20–0.61) | 0.38 (0.22–0.60) | 0.35 (0.19–0.61) | 0.441 |
| Iron prescription, n (%) | 186 (64.4) | 66 (68.8) | 120 (62.2) | 0.299 |
| Iron dose (mg/week) | 50.0 (25.0–60.0) | 50 (25–60) | 35 (25–57.5) | 0.606 |
| Most common home medication | ||||
| Statins, n (%) | 157 (54.3%) | 49 (51.0%) | 108 (56.0%) | 0.454 |
| Antihypertensives, n (%) | 126 (43.6%) | 51 (53.1%) | 75 (38.9%) | 0.024 |
| Antidiabetics, n (%) | 112 (38.8%) | 31 (32.3%) | 81 (42.0%) | 0.125 |
| Biochemical and Dialysis Markers | ||||
| Sodium, mEq/L | 137 (135–139) | 137 (135–139) | 137 (136–139) | 0.610 |
| Potassium, mEq/L | 5.17 ± 0.72 | 5.23 ± 0.74 | 5.15 ± 0.72 | 0.339 |
| Phosphorus, mg/dL | 4.18 (3.35–5.00) | 4.90 (4.12–5.83) | 3.90 (3.30–4.61) | <0.001 |
| Calcium, mg/dL | 9.10 ± 0.59 | 8.98 ± 0.65 | 9.16 ± 0.55 | 0.014 |
| Parathormone, pg/mL | 338 (203–502) | 359 (193–584) | 327 (206–471) | 0.600 |
| Uric acid, mg/dL | 6.45 ± 1.44 | 6.99 ± 1.58 | 6.18 ± 1.28 | <0.001 |
| Creatinine, mg/dL | 8.09 ± 2.34 | 9.17 ± 2.60 | 7.55 ± 2.00 | <0.001 |
| Albumin, g/dL | 3.8 (3.6–4.1) | 3.9 (3.7–4.1) | 3.8 (3.6–4.0) | 0.008 |
| Urea, mg/dL | 116 (97–145) | 122 (106–160) | 111 (92–141) | <0.001 |
| URR, % | 79.0 (76.0–83.0) | 78.0 (75.3–82.0) | 80.0 (76.0–83.0) | 0.215 |
| eKt/V | 1.63 ± 0.29 | 1.65 ± 0.32 | 1.62 ± 0.28 | 0.435 |
| Ultrafiltration volume, L | 2.3 (1.7–2.9) | 2.7 (2.0–3.1) | 2.2 (1.6–2.8) | <0.001 |
| Total (n = 289) | Younger (n = 96) | Older (n = 193) | p | |
|---|---|---|---|---|
| Hematological Data | ||||
| Erythrocytes (×1012/L) | 3.72 (3.44–4.01) | 3.75 (3.55–3.98) | 3.71 (3.43–4.01) | 0.397 |
| Hemoglobin (g/dL) | 11.4 (10.7–12.2) | 11.5 (10.8–12.3) | 11.4 (10.6–12.2) | 0.524 |
| Hematocrit (%) | 35.1 (33.0–37.2) | 35.2 (33.4–37.0) | 35.1 (32.8–37.4) | 0.984 |
| Reticulocytes (×109/L) | 40.1 (26.2–57.8) | 42.0 (29.7–58.8) | 38.9 (25.5–57.4) | 0.276 |
| Platelets (×109/L) | 197 (161–233) | 198 (164–240) | 196 (160–232) | 0.546 |
| Leukocytes (×109/L) | 6.3 (5.3–7.6) | 6.3 (5.4–8.0) | 6.4 (5.3–7.6) | 0.344 |
| Iron metabolism markers | ||||
| Iron (µg/dL) | 55.5 (45.0–74.0) | 56.0 (46.0–75.0) | 54.0 (45.0–74.0) | 0.513 |
| Transferrin (mg/dL) | 183.0 (165.0–210.5) | 185.0 (167.0–213.0) | 180.0 (164.0–209.0) | 0.464 |
| Transferrin saturation (%) | 21.9 (16.8–28.5) | 21.7 (17.4–29.0) | 22.0 (16.7–27.6) | 0.642 |
| sTfR (nmol/L) | 21.6 (16.8–28.0) | 22.8 (18.2–28.1) | 20.6 (16.1–27.9) | 0.105 |
| Ferritin (ng/mL) | 342.0 (213.0–484.5) | 336.0 (183.0–457.5) | 342.0 (218.5–503.0) | 0.251 |
| Hepcidin (ng/mL) | 78.4 (40.9–131.6) | 78.7 (31.8–116.4) | 77.5 (43.4–138.0) | 0.264 |
| Endothelial function/fibrinolysis markers | ||||
| tPA (ng/mL) | 4.2 (3.0–6.1) | 3.4 (2.1–5.5) | 4.4 (3.4–6.3) | <0.001 |
| PAI-1 (ng/mL) | 7.6 (4.7–11.6) | 8.1 (4.2–12.9) | 7.4 (4.9–10.8) | 0.651 |
| D-dimer (ng/mL) | 518.0 (364.0–929.5) | 397.0 (264.3–705.3) | 654.0 (400.0–1030.0) | <0.001 |
| ADMA (µM) | 1.06 (0.89–1.29) | 1.09 (0.91–1.29) | 1.06 (0.86–1.28) | 0.445 |
| Inflammatory markers | ||||
| PTX3 (ng/mL) | 1.40 (0.99–2.05) | 1.28 (0.99–1.85) | 1.42 (0.98–2.11) | 0.490 |
| hsCRP (mg/dL) | 0.37 (0.16–0.81) | 0.37 (0.13–0.77) | 0.38 (0.17–0.85) | 0.409 |
| IL-6 (pg/mL) | 4.10 (2.69–7.33) | 3.61 (2.42–5.93) | 4.63 (3.00–7.62) | 0.003 |
| TNF-α (pg/mL) | 3.38 (2.66–4.59) | 3.03 (2.61–4.33) | 3.49 (2.70–4.70) | 0.066 |
| Lipid profile and adipokines | ||||
| Total cholesterol (mg/dL) | 160.0 (135.0–188.0) | 168.0 (136.0–200.3) | 156.0 (134.5–185.0) | 0.085 |
| Triglycerides (mg/dL) | 133.0 (98.0–185.5) | 137.5 (98.3–202.8) | 129.0 (97.0–173.0) | 0.153 |
| HDL-C (mg/dL) | 45.3 (38.3–55.7) | 44.5 (36.3–56.5) | 45.5 (39.0–54.4) | 0.888 |
| LDL-C (mg/dL) | 79.7 (64.5–107.8) | 83.8 (63.6–109.7) | 79.4 (64.5–106.3) | 0.775 |
| oxLDL (U/L) | 44.8 (35.3–57.3) | 45.8 (36.2–58.6) | 44.6 (34.1–56.8) | 0.623 |
| oxLDL/LDL-C ratio | 0.054 (0.046–0.067) | 0.053 (0.044–0.070) | 0.055 (0.047–0.066) | 0.820 |
| Adiponectin (µg/mL) | 12.20 (7.83–19.23) | 12.19 (7.72–19.34) | 12.20 (7.89–19.16) | 0.877 |
| Leptin (ng/mL) | 15.14 (5.11–45.35) | 12.33 (3.71–33.43) | 17.87 (6.03–49.98) | 0.033 |
| Cardiac marker | ||||
| NT-proBNP (ng/mL) | 13.55 (8.29–24.73) | 10.16 (6.17–17.79) | 15.93 (9.36–25.87) | <0.001 |
| Renal fibrosis marker | ||||
| TIMP-1 (ng/mL) | 535.0 (468.5–619.5) | 516.0 (447.5–575.8) | 543.0 (476.0–642.5) | 0.008 |
| Variable | All Patients (n = 289) | Females (n = 131) | Males (n = 158) | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| DBP | −0.562 | <0.0001 | −0.587 | <0.0001 | −0.547 | <0.0001 |
| tPA | 0.380 | <0.0001 | 0.482 | <0.0001 | 0.301 | 0.0001 |
| D-dimer | 0.367 | <0.0001 | 0.435 | <0.0001 | 0.321 | <0.0001 |
| Phosphorus | −0.352 | <0.0001 | −0.327 | 0.0001 | −0.380 | <0.0001 |
| Creatinine | −0.339 | <0.0001 | −0.297 | 0.0006 | −0.385 | <0.0001 |
| Uric acid | −0.319 | <0.0001 | −0.203 | 0.0200 | −0.381 | <0.0001 |
| NT-proBNP | 0.279 | <0.0001 | 0.186 | 0.0339 | 0.352 | <0.0001 |
| UF volume | −0.261 | <0.0001 | −0.273 | 0.0016 | −0.235 | 0.0030 |
| Albumin | −0.255 | <0.0001 | −0.230 | 0.0083 | −0.266 | 0.0007 |
| TIMP-1 | 0.227 | 0.0001 | 0.314 | 0.0003 | 0.153 | 0.0545 |
| IL-6 | 0.215 | 0.0002 | 0.266 | 0.0022 | 0.193 | 0.0150 |
| Urea | −0.205 | 0.0005 | −0.205 | 0.0188 | −0.193 | 0.0149 |
| Triglycerides | −0.157 | 0.0073 | 0.060 | 0.4946 | −0.305 | 0.0001 |
| Dependent Variable | Model | Unstandardized Coefficients | Standardized Coefficients | t | p | |
|---|---|---|---|---|---|---|
| B | Std. Error | Beta | ||||
| Age (y) | (Constant) | 99.215 | 4.215 | 23.539 | <0.001 | |
| DBP | −0.427 | 0.049 | −0.407 | −8.709 | <0.001 | |
| Ln tPA | 1.243 | 0.212 | 0.272 | 5.865 | <0.001 | |
| Ln Phosphorus | −2.606 | 0.523 | −0.230 | −4.986 | <0.001 | |
| Ln D-dimer | 0.002 | 0.001 | 0.197 | 4.200 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belo, L.; Valente, M.J.; Rocha, S.; Coimbra, S.; Catarino, C.; Lousa, I.; Bronze-da-Rocha, E.; Rocha-Pereira, P.; Sameiro-Faria, M.d.; Oliveira, J.G.; et al. Age-Related Changes in Clinical and Analytical Variables in Chronic Hemodialyzed Patients. Int. J. Mol. Sci. 2024, 25, 3325. https://doi.org/10.3390/ijms25063325
Belo L, Valente MJ, Rocha S, Coimbra S, Catarino C, Lousa I, Bronze-da-Rocha E, Rocha-Pereira P, Sameiro-Faria Md, Oliveira JG, et al. Age-Related Changes in Clinical and Analytical Variables in Chronic Hemodialyzed Patients. International Journal of Molecular Sciences. 2024; 25(6):3325. https://doi.org/10.3390/ijms25063325
Chicago/Turabian StyleBelo, Luís, Maria João Valente, Susana Rocha, Susana Coimbra, Cristina Catarino, Irina Lousa, Elsa Bronze-da-Rocha, Petronila Rocha-Pereira, Maria do Sameiro-Faria, José Gerardo Oliveira, and et al. 2024. "Age-Related Changes in Clinical and Analytical Variables in Chronic Hemodialyzed Patients" International Journal of Molecular Sciences 25, no. 6: 3325. https://doi.org/10.3390/ijms25063325