Alkylbenzoic and Alkyloxybenzoic Acid Blending for Expanding the Liquid Crystalline State and Improving Its Rheology
Abstract
:1. Introduction
2. Results and Discussion
2.1. A Blend of Alkylbenzoic Acids (4BA/6BA)
2.2. A Blend of Alkyloxybenzoic Acids (4OBA/5OBA)
2.3. Blends of Alkylbenzoic and Alkyloxybenzoic Acids
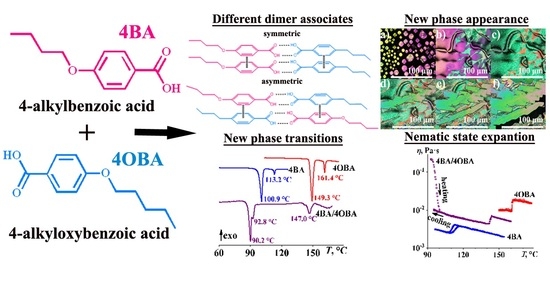
2.3.1. Effect of Molecular Length Ratio (4BA/4OBA, 4BA/5OBA, 7BA/4OBA, and 7BA/5OBA)
2.3.2. Effect of Substituent Length (6BA/4OBA, 6BA/5OBA, 6BA/8OBA, 6BA/9OBA, 6BA/12OBA, and 6BA/16OBA)
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
- The blending of alkylbenzoic acids or/and alkyloxybenzoic acids causes the formation of new asymmetric heterodimers that form a mesophase, just like these acids’ homodimers but with different transition temperatures and different viscosity properties.
- The blend of alkylbenzoic acids has a new monotropic transition to the second nematic phase, which is not accompanied by a change in viscosity, whereas that of alkyloxybenzoic acids has an enantiotropic transition to the new smectic phase having a very high viscosity.
- The blending of alkylbenzoic and alkyloxybenzoic acids probably leads to the simultaneous formation of symmetric and asymmetric associates (more and less high-melting, respectively) from their asymmetric dimers, at least the crystal–nematic and nematic–isotropic transitions in some cases occur in two-step manner according to the calorimetry data.
- High-viscosity flow of the kBA/kOBA blends at the crystal–nematic transition is possible, even if a part of symmetric associates is in the crystalline state; however, for the realization of the low-viscous nematic state, the metamorphosis of both forms of associates into the nematic phase is necessary.
- For the transition of an isotropic liquid to the nematic state, the formation of asymmetric associates is necessary (as formed at lower temperatures), whereas their loss of nematic ordering destroys the symmetric associates’ order and makes the blend an isotropic liquid.
- The blending of alkylbenzoic and alkyloxybenzoic acids with short radicals (butyl- or pentyl-) leads to a new mono- or enantiotropic transition to the second nematic phase without changing the viscosity at this evolution.
- The blend of alkylbenzoic and alkyloxybenzoic acids with the same molecular lengths has distinct transitions of symmetric and asymmetric associates between their crystalline and nematic states. A decrease in the alkyloxybenzoic acid’s molecular size leads to a broadening of the melting region of the symmetric associates, while its increase causes a two-step or even blurred isotropic–nematic transition, which is possibly due to the transformation of initially formed symmetric associates into the asymmetric ones.
- The blending of smectogenic and nematogenic acids can suppress the formation of the smectic phase if its temperature range for smectogenic molecules is low, whereas the nematic state forms even if one of the acids is not a nematogen.
- The blending of p-n-hexylbenzoic and p-n-octyloxybenzoic acids forms a mesogen having the most extended temperature range for a nematic state, including low temperatures (from 57 °C to 133 °C, 76 °C in difference). In turn, the mixing of p-n-butyloxybenzoic acid and p-n-pentyloxybenzoic acid gives the highest-temperature nematic phase with the broadest existence region (up to 156 °C), while the combination of p-n-hexylbenzoic acid and p-n-nonyloxy- or p-n-dodecyloxybenzoic acid results in the lowest temperature smectic phase (down to 51 °C).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priestly, E. Introduction to Liquid Crystals; Priestley, E.B., Wojtowicz, P.J., Sheng, P., Eds.; Springer: Boston, MA, USA, 1975; ISBN 978-1-4684-2177-4. [Google Scholar]
- Khoo, I.-C. Liquid Crystals, 3rd ed.; Wiley Series in Pure and Applied Optics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; ISBN 978-1-119-70582-6. [Google Scholar]
- Dąbrowski, R.; Kula, P.; Herman, J. High Birefringence Liquid Crystals. Crystals 2013, 3, 443–482. [Google Scholar] [CrossRef]
- Wu, S.-T.; Efron, U.; Hess, L.D. Birefringence Measurements of Liquid Crystals. Appl. Opt. 1984, 23, 3911. [Google Scholar] [CrossRef] [PubMed]
- Buka, A.; de Jeu, W.H. Diamagnetism and Orientational Order of Nematic Liquid Crystals. J. Phys. Fr. 1982, 43, 361–367. [Google Scholar] [CrossRef]
- Serra, F.; Buscaglia, M.; Bellini, T. The Emergence of Memory in Liquid Crystals. Mater. Today 2011, 14, 488–494. [Google Scholar] [CrossRef]
- Ważyńska, B.; Okowiak, J.A. Tribological Properties of Nematic and Smectic Liquid Crystalline Mixtures Used as Lubricants. Tribol. Lett. 2006, 24, 1–5. [Google Scholar] [CrossRef]
- Kupchinov, B.I. Tribology and the Liquid-Crystalline State. J. Frict. Wear 2009, 30, 169–171. [Google Scholar] [CrossRef]
- Carrión, F.-J.; Martínez-Nicolás, G.; Iglesias, P.; Sanes, J.; Bermúdez, M.-D. Liquid Crystals in Tribology. Int. J. Mol. Sci. 2009, 10, 4102–4115. [Google Scholar] [CrossRef]
- Figueiredo Neto, A.M.; Salinas, S.R.A. The Physics of Lyotropic Liquid Crystals; Oxford University Press: Oxford, UK, 2005; ISBN 978-0-19-852550-9. [Google Scholar]
- Martin, J.D.; Keary, C.L.; Thornton, T.A.; Novotnak, M.P.; Knutson, J.W.; Folmer, J.C.W. Metallotropic Liquid Crystals Formed by Surfactant Templating of Molten Metal Halides. Nat. Mater. 2006, 5, 271–275. [Google Scholar] [CrossRef]
- Vertogen, G.; De Jeu, W.H. Thermotropic Liquid Crystals, Fundamentals; Springer Science & Business Media: Dordrecht, The Netherlands, 2012; ISBN 978-3-642-83133-1. [Google Scholar]
- Ramamoorthy, A. (Ed.) Thermotropic Liquid Crystals: Recent Advances; Springer: Dordrecht, The Netherlands, 2007; ISBN 978-1-4020-5327-6. [Google Scholar]
- Ilyin, S.O.; Konstantinov, I.I. Rheology of Highly Ordered Smectic Phases Based on Biphenyl Derivatives. J. Mol. Liq. 2022, 363, 119872. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Giesselmann, F. Current Topics in Smectic Liquid Crystal Research. Chem. Phys. Chem. 2006, 7, 20–45. [Google Scholar] [CrossRef]
- Dierking, I.; Mitov, M.; Osipov, M.A. Smectic Layer Instabilities in Liquid Crystals. Soft. Matter. 2015, 11, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Jakli, A.; Saupe, A. One- and Two-Dimensional Fluids; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-1-4200-1220-0. [Google Scholar]
- Oswald, P.; Pieranski, P. Smectic and Columnar Liquid Crystals; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-0-429-12555-3. [Google Scholar]
- Muševič, I. Nematic Liquid-Crystal Colloids. Materials 2017, 11, 24. [Google Scholar] [CrossRef]
- Dunmur, D.; Fukuda, A.; Luckhurst, G. Physical Properties of Liquid Crystals: Nematics; Luckhurst, G.R., Dunmur, D., Fukuda, A., Eds.; EMIS Data Reviews Series; Institution of Engineers: Singapore, 2001; ISBN 978-0-85296-784-3. [Google Scholar]
- Kirsch, P.; Bremer, M. Nematic Liquid Crystals for Active Matrix Displays: Molecular Design and Synthesis. Angew. Chem. Int. Ed. 2000, 39, 4216–4235. [Google Scholar] [CrossRef]
- Coates, D. Development and Applications of Cholesteric Liquid Crystals. Liq. Cryst. 2015, 42, 653–665. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Konstantinov, I.I. Rheological Evidence for the Existence of Subphases in the Liquid Crystalline 4-n-Alkoxybenzoic Acids. Liq. Cryst. 2015, 43, 369–380. [Google Scholar] [CrossRef]
- Martin, S.M.; Yonezawa, J.; Horner, M.J.; Macosko, C.W.; Ward, M.D. Structure and Rheology of Hydrogen Bond Reinforced Liquid Crystals. Chem. Mater. 2004, 16, 3045–3055. [Google Scholar] [CrossRef]
- Cidade, M.T.; Pereira, G.; Bubnov, A.; Hamplova, V.; Kaspar, M.; Casquilho, J.P. Rheological Characterisation of a Liquid-Crystalline Diol and Its Dependence with an Applied Electric Field. Liq. Cryst. 2012, 39, 191–197. [Google Scholar] [CrossRef]
- Bubnov, A.; Cigl, M.; Machado, A.; Pociecha, D.; Hamplova, V.; Cidade, M.T. Design of Calamitic Self-Assembling Reactive Mesogenic Units: Mesomorphic Behaviour and Rheological Characterisation. Liq. Cryst. 2018, 45, 561–573. [Google Scholar] [CrossRef]
- Kulichikhin, V.G.; Kudryavtsev, G.I.; Papkov, S.P. Rheological Properties of Liquid-Crystalline Polymer Solutions. Int. J. Polym. Mater. Polym. Biomater. 1982, 9, 239–256. [Google Scholar] [CrossRef]
- Vshivkov, S.A.; Rusinova, E.V.; Galyas, A.G. Phase Diagrams and Rheological Properties of Cellulose Ether Solutions in Magnetic Field. Eur. Polym. J. 2014, 59, 326–332. [Google Scholar] [CrossRef]
- Kulichikhin, V.G. Rheology, Phase Equilibria and Processing of Lyotropic Liquid Crystalline Polymers. Mol. Cryst. Liq. Cryst. Inc. Nonlinear Opt. 1989, 169, 51–81. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Kotomin, S.V. Mesophase State and Shear-Affected Phase Separation of Poly(p-Phenylene-Benzimidazole-Terephthalamide) Solutions in N,N-Dimethylacetamide. J. Polym. Res. 2022, 29, 326. [Google Scholar] [CrossRef]
- Tamaoki, N. Cholesteric Liquid Crystals for Color Information Technology. Adv. Mater. 2001, 13, 1135–1147. [Google Scholar] [CrossRef]
- O’Neill, M.; Kelly, S.M. Liquid Crystals for Charge Transport, Luminescence, and Photonics. Adv. Mater. 2003, 15, 1135–1146. [Google Scholar] [CrossRef]
- Mori, S.; Iwata, H. Relationship between Tribological Performance of Liquid Crystals and Their Molecular Structure. Tribol. Int. 1996, 29, 35–39. [Google Scholar] [CrossRef]
- Usol’tseva, N.V.; Smirnova, M.V.; Kazak, A.V.; Smirnova, A.I.; Bumbina, N.V.; Ilyin, S.O.; Rozhkova, N.N. Rheological Characteristics of Different Carbon Nanoparticles in Cholesteric Mesogen Dispersions as Lubricant Coolant Additives. J. Frict. Wear 2015, 36, 380–385. [Google Scholar] [CrossRef]
- Avilés, M.D.; Sánchez, C.; Pamies, R.; Sanes, J.; Bermúdez, M.D. Ionic Liquid Crystals in Tribology. Lubricants 2019, 7, 72. [Google Scholar] [CrossRef]
- Usol’tseva, N.V.; Smirnova, A.I. Liquid Crystals as Lubricants. Lubricants 2019, 7, 111. [Google Scholar] [CrossRef]
- Nagaraj, M. Liquid Crystals Templating. Crystals 2020, 10, 648. [Google Scholar] [CrossRef]
- Yadikova, A.E.; Yashchenko, V.S.; Makarova, V.V.; Matveenko, Y.V.; Kostyuk, A.V.; Ilyin, S.O. Synthesis and Properties of Sulfonated Copolymers of Oxadiazole, Dioxophenoxathiine, and Diphenyl Oxide. Polym. Sci. Ser. B 2020, 62, 47–60. [Google Scholar] [CrossRef]
- Nishikori, Y.; Iseda, K.; Kokado, K.; Sada, K. Mesogenic Polyelectrolyte Gels Absorb Organic Solvents and Liquid Crystalline Molecules. Polymers 2016, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Luan, C.; Luan, H.; Luo, D. Application and Technique of Liquid Crystal-Based Biosensors. Micromachines 2020, 11, 176. [Google Scholar] [CrossRef]
- Munna, M.; Anwar, F.; Coutu, R. Nematic Liquid Crystal Composite Materials for DC and RF Switching. Technologies 2019, 7, 32. [Google Scholar] [CrossRef]
- Borodulina, T.; Bermesheva, E.; Smirnova, N.; Ilyin, S.; Brantseva, T.; Antonov, S. Adhesive Properties of Liquid Crystalline Hydroxypropyl Cellulose–Propylene Glycol Blends. J. Adhes. Sci. Technol. 2014, 28, 1629–1643. [Google Scholar] [CrossRef]
- Wen, Z.; Yang, K.; Raquez, J.-M. A Review on Liquid Crystal Polymers in Free-Standing Reversible Shape Memory Materials. Molecules 2020, 25, 1241. [Google Scholar] [CrossRef]
- Ku, K.; Hisano, K.; Yuasa, K.; Shigeyama, T.; Akamatsu, N.; Shishido, A.; Tsutsumi, O. Effect of Crosslinkers on Optical and Mechanical Behavior of Chiral Nematic Liquid Crystal Elastomers. Molecules 2021, 26, 6193. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Yadykova, A.Y.; Makarova, V.V.; Yashchenko, V.S.; Matveenko, Y.V. Sulfonated Polyoxadiazole Synthesis and Processing into Ion-conducting Films. Polym. Int. 2020, 69, 1243–1255. [Google Scholar] [CrossRef]
- Miyagi, K.; Teramoto, Y. Construction of Functional Materials in Various Material Forms from Cellulosic Cholesteric Liquid Crystals. Nanomaterials 2021, 11, 2969. [Google Scholar] [CrossRef]
- Shibaev, V.P. Liquid-Crystalline Polymer Systems: From the Past to the Present. Polym. Sci. Ser. A 2014, 56, 727–762. [Google Scholar] [CrossRef]
- Kaafarani, B.R. Discotic Liquid Crystals for Opto-Electronic Applications. Chem. Mater. 2011, 23, 378–396. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tsiourvas, D. Supramolecular Hydrogen-Bonded Liquid Crystals. Liq. Cryst. 2001, 28, 1127–1161. [Google Scholar] [CrossRef]
- Okumuş, M.; Özgan, Ş. Thermal and Mesomorphic Properties of Ternary Mixtures of Some Hydrogen-Bonded Liquid Crystals. Liq. Cryst. 2014, 41, 1293–1302. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Naoum, M.M. Mesophase Behavior of Binary and Ternary Mixtures of Benzoic Acids Bearing Terminal Substituents of Different Polarity and Chain-Lengths. Thermochim. Acta 2014, 575, 122–128. [Google Scholar] [CrossRef]
- Kang, S.K.; Samulski, E.T. Liquid Crystals Comprising Hydrogen-Bonded Organic Acids I. Mixtures of Non-Mesogenic Acids. Liq. Cryst. 2000, 27, 371–376. [Google Scholar] [CrossRef]
- Lohar, J.M. A study of mixed liquid crystal formation in mixture of p-methoxy and p-ethoxy benzoic acids. J. Phys. Colloq. 1975, 36, 399–401. [Google Scholar] [CrossRef]
- Miranda, M.D.; Chávez, F.V.; Maria, T.M.R.; Eusébio, M.E.S.; Sebastião, P.J.; Martín-Ramos, P.; Figueirinhas, J.L.; Silva, M.R. Synthesis of Liquid Crystals Based on Hydrogen-Bonding of 4-(Octyloxy)Benzoic Acid with 4-Alkylbenzoic Acids. Mol. Crys. Liq. Cryst. 2016, 630, 87–101. [Google Scholar] [CrossRef]
- Kavitha, C.; Prabu, N.P.S.; Madhu Mohan, M.L.N. Thermal Analysis of Supramolecular Hydrogen-Bonded Liquid Crystals Formed by Nonyloxy and Alkyl Benzoic Acids. Mol. Crys. Liq. Cryst. 2013, 574, 96–113. [Google Scholar] [CrossRef]
- Kuz’mina, L.G.; Kucherepa, N.S.; Churakov, A.V. Mesophase Design: II. Molecular Structure and Crystal Packing of 4-Alkyloxycyanobiphenyls. Crystallogr. Rep. 2012, 57, 213–226. [Google Scholar] [CrossRef]
- Kuz’mina, L.G.; Navasardyan, M.A.; Churakov, A.V.; Howard, J.A.K. X-Ray Diffraction Study of n-(Alkyloxybenzilidene)-n’-Toluidines. Mol. Cryst. Liq. Cryst. 2016, 638, 60–67. [Google Scholar] [CrossRef]
- Konstantinov, I.I.; Churakov, A.V.; Kuz’mina, L.G. Molecular and Crystal Structures of 4-Acylphenyl 4′-Alkyloxybenzoates. Crystallogr. Rep. 2013, 58, 81–92. [Google Scholar] [CrossRef]
- Kuz’mina, L.G.; Kucherepa, N.S.; Pestov, S.M.; Kochetov, A.N.; Rukk, N.S.; Syrbu, S.A. Molecular and Crystal Structure of 4-Alkoxybenzoic Acids: Design of the Mesogenic Phase. Crystallogr. Rep. 2009, 54, 862–879. [Google Scholar] [CrossRef]
- Kuz’mina, L.G.; Kucherepa, N.S. Mesophase Design: I. Molecular Structure and Crystal Packing Motifs of 4-Alkylcyanobiphenyls. Crystallogr. Rep. 2011, 56, 242–255. [Google Scholar] [CrossRef]
- Meille, S.V.; Allegra, G.; Geil, P.H.; He, J.; Hess, M.; Jin, J.-I.; Kratochvíl, P.; Mormann, W.; Stepto, R. Definitions of Terms Relating to Crystalline Polymers (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1831–1871. [Google Scholar] [CrossRef]
- Clarke, J.B.; Hastie, J.W.; Kihlborg, L.H.E.; Metselaar, R.; Thackeray, M.M. Definitions of Terms Relating to Phase Transitions of the Solid State (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 577–594. [Google Scholar] [CrossRef]
- Barón, M. Definitions of Basic Terms Relating to Low-Molar-Mass and Polymer Liquid Crystals (IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 845–895. [Google Scholar] [CrossRef]
- Savins, J.G.; Metzner, A.B. Radial (Secondary) Flows in Rheogoniometric Devices. Rheol. Acta 1970, 9, 365–373. [Google Scholar] [CrossRef]
- Fewell, M.E.; Hellums, J.D. The Secondary Flow of Newtonian Fluids in Cone-and-Plate Viscometers. Trans. Soc. Rheol. 1977, 21, 535–565. [Google Scholar] [CrossRef]
- Bennett, G.M.; Jones, B. Mesomorphism and Polymorphism of Some p-Alkoxybenzoic and p-Alkoxycinnamic Acids. J. Chem. Soc. 1939, 420–425. [Google Scholar] [CrossRef]
- Pérez, E.M.; Martín, N. π–π Interactions in Carbon Nanostructures. Chem. Soc. Rev. 2015, 44, 6425–6433. [Google Scholar] [CrossRef]
- Claessens, C.G.; Stoddart, J.F. π–π Interactions in self-assembly. J. Phys. Org. Chem. 1997, 10, 254–272. [Google Scholar] [CrossRef]
- Schramm, G.A. Practical Approach to Rheology and Rheometry; Haake: Karlsruhe, Germany, 1994. [Google Scholar]
- Strelets, L.A.; Ilyin, S.O. Effect of Enhanced Oil Recovery on the Composition and Rheological Properties of Heavy Crude Oil. J. Petrol. Sci. Eng. 2021, 203, 108641. [Google Scholar] [CrossRef]































Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadykova, A.Y.; Konstantinov, I.I.; Vlasova, A.V.; Varfolomeeva, L.A.; Ilyin, S.O. Alkylbenzoic and Alkyloxybenzoic Acid Blending for Expanding the Liquid Crystalline State and Improving Its Rheology. Int. J. Mol. Sci. 2023, 24, 15706. https://doi.org/10.3390/ijms242115706
Yadykova AY, Konstantinov II, Vlasova AV, Varfolomeeva LA, Ilyin SO. Alkylbenzoic and Alkyloxybenzoic Acid Blending for Expanding the Liquid Crystalline State and Improving Its Rheology. International Journal of Molecular Sciences. 2023; 24(21):15706. https://doi.org/10.3390/ijms242115706
Chicago/Turabian StyleYadykova, Anastasiya Y., Ivan I. Konstantinov, Anna V. Vlasova, Lydia A. Varfolomeeva, and Sergey O. Ilyin. 2023. "Alkylbenzoic and Alkyloxybenzoic Acid Blending for Expanding the Liquid Crystalline State and Improving Its Rheology" International Journal of Molecular Sciences 24, no. 21: 15706. https://doi.org/10.3390/ijms242115706