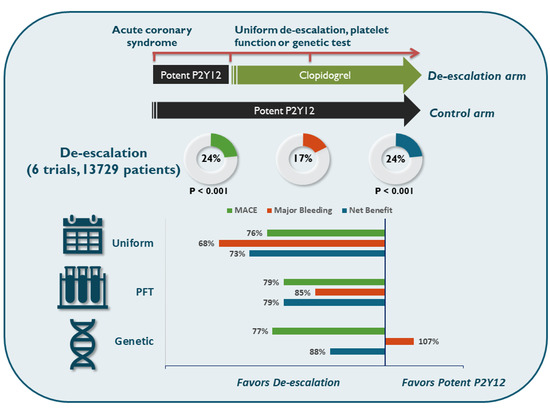
Individualized or Uniform De-Escalation Strategies for Antiplatelet Therapy in Acute Coronary Syndrome: A Review of Clinical Trials with Platelet Function Testing and Genetic Testing-Based Protocols
Abstract
:1. Introduction
2. Pathophysiological Background
3. Role of Platelet Function Testing in Assessing P2Y12 Inhibitor Therapy
4. Genetic Background of Interindividual Response Variability by Clopidogrel
5. The Use of P2Y12 Inhibitors in Acute Coronary Syndrome
6. Genetic Testing-Based P2Y12 De-Escalation Strategy
7. Platelet Function Testing-Based P2Y12 De-Escalation Strategy
8. Trials with Uniform P2Y12 De-Escalation Strategy
9. Comparison of Approaches
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Lopes, R.D.; Harrington, R.A. Diagnosis and Treatment of Acute Coronary Syndromes: A Review. JAMA 2022, 327, 662–675. [Google Scholar] [CrossRef]
- Gimbel, M.E.; Minderhoud, S.C.S.; ten Berg, J.M. A practical guide on how to handle patients with bleeding events while on oral antithrombotic treatment. Neth. Heart J. 2018, 26, 341–351. [Google Scholar] [CrossRef]
- Howell, S.J.; Hoeks, S.E.; West, R.M.; Wheatcroft, S.B.; Hoeft, A.; OBTAIN Investigators of European Society of Anaesthesiology (ESA) Clinical Trial Network. Prospective observational cohort study of the association between antiplatelet therapy, bleeding and thrombosis in patients with coronary stents undergoing noncardiac surgery. Br. J. Anaesth. 2019, 122, 170–179. [Google Scholar] [CrossRef]
- Khodadi, E. Platelet Function in Cardiovascular Disease: Activation of Molecules and Activation by Molecules. Cardiovasc. Toxicol. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Orban, M.; Sibbing, D. Platelet function testing in patients with acute coronary syndrome. J. Cardiovasc. Transl. Res. 2013, 6, 371–377. [Google Scholar] [CrossRef]
- Hechler, B.; Gachet, C. P2 receptors and platelet function. Purinergic Signal 2011, 7, 293–303. [Google Scholar] [CrossRef]
- Born, G. Platelets: Past, present and future. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 210. [Google Scholar] [CrossRef]
- Morrow, D.A.; Scirica, B.M.; Fox, K.A.; Berman, G.; Strony, J.; Veltri, E.; Bonaca, M.P.; Fish, P.; McCabe, C.H.; Braunwald, E. Evaluation of a novel antiplatelet agent for secondary prevention in patients with a history of atherosclerotic disease: Design and rationale for the Thrombin-Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P)-TIMI 5. Am. Heart J. 2009, 158, 335–341.e3. [Google Scholar] [CrossRef] [PubMed]
- Tra, T.; Executive, C.E.R.; Committees, S. The Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRA•CER) trial: Study design and rationale. Am. Heart J. 2009, 158, 327–334.e4. [Google Scholar] [CrossRef]
- Aradi, D.; Komócsi, A.; Vorobcsuk, A.; Rideg, O.; Tőkés-Füzesi, M.; Magyarlaki, T.; Horváth, I.G.; Serebruany, V.L. Prognostic significance of high on-clopidogrel platelet reactivity after percutaneous coronary intervention: Systematic review and meta-analysis. Am. Heart J. 2010, 160, 543–551. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef]
- James, S.; Åkerblom, A.; Cannon, C.P.; Emanuelsson, H.; Husted, S.; Katus, H.; Skene, A.; Steg, P.G.; Storey, R.F.; Harrington, R.; et al. Comparison of ticagrelor, the first reversible oral P2Y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: Rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am. Heart J. 2009, 157, 599–605. [Google Scholar] [CrossRef]
- Lordkipanidzé, M. Platelet function tests. Semin. Thromb. Hemost. 2016, 42, 258–267. [Google Scholar] [CrossRef]
- Harrison, P.; Lordkipanidzé, M. Testing platelet function. Hematol. Oncol. Clin. N. Am. 2013, 27, 411–441. [Google Scholar] [CrossRef]
- Koltai, K.; Kesmarky, G.; Feher, G.; Tibold, A.; Toth, K. Platelet aggregometry testing: Molecular mechanisms, techniques and clinical implications. Int. J. Mol. Sci. 2017, 18, 1803. [Google Scholar] [CrossRef]
- Sibbing, D.; Aradi, D.; Alexopoulos, D.; Berg, J.T.; Bhatt, D.L.; Bonello, L.; Collet, J.-P.; Cuisset, T.; Franchi, F.; Gross, L.; et al. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y12 Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1521–1537. [Google Scholar] [CrossRef]
- Lewis, J.P.; Backman, J.D.; Reny, J.-L.; Bergmeijer, T.O.; Mitchell, B.D.; Ritchie, M.D.; Déry, J.-P.; Pakyz, R.E.; Gong, L.; Ryan, K.; et al. Pharmacogenomic polygenic response score predicts ischaemic events and cardiovascular mortality in clopidogrel-treated patients. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 203–210, Erratum in Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 269. [Google Scholar] [CrossRef]
- Rideg, O.; Komócsi, A.; Magyarlaki, T.; Tőkés-Füzesi, M.; Miseta, A.; Kovács, G.L.; Aradi, D. Impact of genetic variants on post-clopidogrel platelet reactivity in patients after elective percutaneous coronary intervention. Pharmacogenomics 2011, 12, 1269–1280. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- Price, M.J.; Angiolillo, D.J.; Teirstein, P.S.; Lillie, E.; Manoukian, S.V.; Berger, P.B.; Tanguay, J.-F.; Cannon, C.P.; Topol, E. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: A time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial. Circulation 2011, 124, 1132–1137. [Google Scholar] [CrossRef]
- Collet, J.-P.; Hulot, J.-S.; Cuisset, T.; Rangé, G.; Cayla, G.; VAN Belle, E.; Elhadad, S.; Rousseau, H.; Sabouret, P.; O’Connor, S.A.; et al. Genetic and platelet function testing of antiplatelet therapy for percutaneous coronary intervention: The ARCTIC-GENE study. Eur. J. Clin. Pharmacol. 2015, 71, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Claassens, D.M.F.; Vos, G.J.; Bergmeijer, T.O.; Hermanides, R.S.; Van’t Hof, A.W.; Van Der Harst, P.; Barbato, E.; Morisco, C.; Gin, R.M.T.J.; Asselbergs, F.W.; et al. A Genotype-Guided Strategy for Oral P2Y 12 Inhibitors in Primary PCI. N. Engl. J. Med. 2019, 381, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Capodanno, D.; Baber, U.; Bhatt, D.L.; Collet, J.-P.; Dangas, G.; Franchi, F.; Gibson, C.M.; Gwon, H.-C.; Kastrati, A.; Kimura, T.; et al. P2Y12 inhibitor monotherapy in patients undergoing percutaneous coronary intervention. Nat. Rev. Cardiol. 2022, 19, 829–844. [Google Scholar] [CrossRef]
- Schüpke, S.; Neumann, F.-J.; Menichelli, M.; Mayer, K.; Bernlochner, I.; Wöhrle, J.; Richardt, G.; Liebetrau, C.; Witzenbichler, B.; Antoniucci, D.; et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2019, 381, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.L.; Farkouh, M.E.; So, D.; Lennon, R.; Geller, N.; Mathew, V.; Bell, M.; Bae, J.-H.; Jeong, M.H.; Chavez, I.; et al. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs Conventional Clopidogrel Therapy on Ischemic Outcomes after Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial. JAMA 2020, 324, 761–771. [Google Scholar] [CrossRef]
- Sibbing, D.; Aradi, D.; Jacobshagen, C.; Gross, L.; Trenk, D.; Geisler, T.; Orban, M.; Hadamitzky, M.; Merkely, B.; Kiss, R.G.; et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): A randomised, open-label, multicentre trial. Lancet 2017, 390, 1747–1757. [Google Scholar] [CrossRef]
- Cuisset, T.; Deharo, P.; Quilici, J.; Johnson, T.W.; Deffarges, S.; Bassez, C.; Bonnet, G.; Fourcade, L.; Mouret, J.P.; Lambert, M.; et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: The TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur. Heart J. 2017, 38, 3070–3078. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kang, J.; Hwang, D.; Han, J.-K.; Yang, H.-M.; Kang, H.-J.; Koo, B.-K.; Rhew, J.Y.; Chun, K.-J.; Lim, Y.-H.; et al. Prasugrel-based de-escalation of dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (HOST-REDUCE-POLYTECH-ACS): An open-label, multicentre, non-inferiority randomised trial. Lancet 2020, 396, 1079–1089. [Google Scholar] [CrossRef]
- Park, M.-W.; Kim, C.J.; Kim, M.-C.; Choo, E.-H.; Hwang, B.-H.; Park, C.-S.; Kim, H.-Y.; Yoo, K.-D.; Jeon, D.-S.; Jeong, M.H.; et al. A prospective, multicentre, randomised, open-label trial to compare the efficacy and safety of clopidogrel versus ticagrelor in stabilised patients with acute myocardial infarction after percutaneous coronary intervention: Rationale and design of the TALOS-AMI trial. EuroIntervention 2021, 16, 1170–1176. [Google Scholar] [CrossRef]
- Ueno, T.; Koiwaya, H.; Sasaki, K.-I.; Katsuki, Y.; Katsuda, Y.; Murasato, Y.; Shimamatsu, J.; Umeji, K.; Otsuka, Y.; Kawasaki, T.; et al. Changes in P2Y12 reaction units after switching treatments from prasugrel to clopidogrel in Japanese patients with acute coronary syndrome followed by elective coronary stenting. Cardiovasc. Interv. Ther. 2017, 32, 341–350. [Google Scholar] [CrossRef]
- El Alaoui El Abdallaoui, O.; Tornyos, D.; Lukács, R.; Komócsi, A. Abatement of potent P2Y12 antagonist-based dual antiplatelet therapy after coronary intervention: A network meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2023, 9, 1008914. [Google Scholar] [CrossRef] [PubMed]
- Kuno, T.; Fujisaki, T.; Shoji, S.; Sahashi, Y.; Tsugawa, Y.; Iwagami, M.; Takagi, H.; Briasoulis, A.; Deharo, P.; Cuisset, T.; et al. Comparison of Unguided De-Escalation Versus Guided Selection of Dual Antiplatelet Therapy After Acute Coronary Syndrome: A Systematic Review and Network Meta-Analysis. Circ. Cardiovasc. Interv. 2022, 15, e011990. [Google Scholar] [CrossRef] [PubMed]




| Study | TALOS-AMI Trial | HOST-REDUCE-POLYTECH-ACS | TAILOR-PCI | TOPIC | TROPICAL-ACS | - |
|---|---|---|---|---|---|---|
| First author | Park | Kim | Pereira | Cuisset | Sibbing | Ueno |
| Publication year | 2021 | 2020 | 2020 | 2017 | 2017 | 2016 |
| Number of patients | 2697 | 2338 | 5302 | 646 | 2610 | 136 |
| De-escalation strategy | Uniform unguided de-escalation | Uniform unguided de-escalation | Genotype-guided therapy | Uniform unguided de-escalation | Guided by platelet function testing | Uniform unguided de-escalation |
| Primary outcome | NACE (CVD + MI + Stroke + Bleeding) | NACE (Death + MI + ST + SRI + Bleeding) | CVD + MI + ST + RR + Stroke | CVD + UR + Stroke + Bleeding | CVD + MI + Stroke + Bleeding | PRU |
| Definition of bleeding (Primary/Secondary) | BARC | BARC | BARC/TIMI | TIMI/BARC | BARC | BARC/TIMI |
| Treatment used before de-escalation | Ticagrelor + Aspirin | Prasugrel + Aspirin | Ticagrelor + Aspirin | Ticagrelor or Prasugrel + Aspirin | Prasugrel + Aspirin | Prasugrel + Aspirin |
| Treatment used after de-escalation | Clopidogrel + Aspirin | Prasugrel + Aspirin | Clopidogrel + Aspirin | Clopidogrel + Aspirin | Clopidogrel + Aspirin | Clopidogrel + Aspirin |
| Clopidogrel (Experimental/Control) (%) | 100/0 | - | 15/99 | 100/0 | 100/0 | 100/0 |
| Prasugrel(Experimental/Control) (%) | 0/100 | 100/100 | - | 56/59 | 0/100 | 0/100 |
| Ticagrelor (Experimental/Control) (%) | 0/100 | - | 85/1 | 44/42 | - | - |
| Result | Significant decrease in bleeding risk | Reduced risk of NACE | No significant results | Reduced risk of bleeding | No significant results | Increase in PRU |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Abdallaoui, O.E.A.; Tornyos, D.; Lukács, R.; Szabó, D.; Komócsi, A. Individualized or Uniform De-Escalation Strategies for Antiplatelet Therapy in Acute Coronary Syndrome: A Review of Clinical Trials with Platelet Function Testing and Genetic Testing-Based Protocols. Int. J. Mol. Sci. 2023, 24, 9071. https://doi.org/10.3390/ijms24109071
El Abdallaoui OEA, Tornyos D, Lukács R, Szabó D, Komócsi A. Individualized or Uniform De-Escalation Strategies for Antiplatelet Therapy in Acute Coronary Syndrome: A Review of Clinical Trials with Platelet Function Testing and Genetic Testing-Based Protocols. International Journal of Molecular Sciences. 2023; 24(10):9071. https://doi.org/10.3390/ijms24109071
Chicago/Turabian StyleEl Abdallaoui, Oumaima El Alaoui, Dániel Tornyos, Réka Lukács, Dóra Szabó, and András Komócsi. 2023. "Individualized or Uniform De-Escalation Strategies for Antiplatelet Therapy in Acute Coronary Syndrome: A Review of Clinical Trials with Platelet Function Testing and Genetic Testing-Based Protocols" International Journal of Molecular Sciences 24, no. 10: 9071. https://doi.org/10.3390/ijms24109071