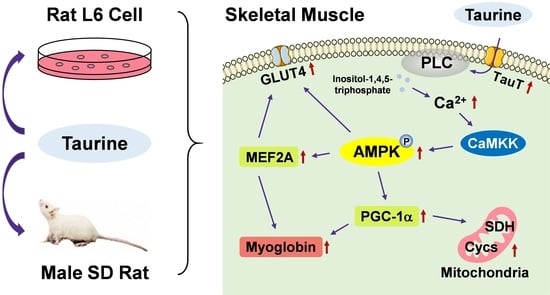
Taurine Stimulates AMP-Activated Protein Kinase and Modulates the Skeletal Muscle Functions in Rats via the Induction of Intracellular Calcium Influx
Abstract
:1. Introduction
2. Results
2.1. Effects of Taurine Administration on Taurine Levels in the Plasma and Skeletal Muscles of SD Rats
2.2. Effects of Taurine on the Expression Levels of Myogenic Genes in the Skeletal Muscles of SD Rats
2.3. Effects of Taurine on the Phosphorylation of AMPK and the Expression Levels of Myogenic Proteins
2.4. Effects of Taurine Administration on Mitochondrial DNA (mtDNA) and SDH Staining in the Skeletal Muscles of SD Rats
2.5. Effects of Taurine on the Expression Levels of Myogenic Genes in L6 Cells
2.6. Taurine Induces the Phosphorylation of AMPK in L6 Myotubes
2.7. Effects of Taurine Transporter Antagonist, Guanidinoethyl Sulfonate (GES), and AMPK Inhibitor, Adenine 9-β-D-arabinofuranoside (araA), on the Phosphorylation of AMPK and Expression Levels of Myogenic Genes and Proteins
2.8. Induction of Intracellular Calcium Influx after Treatment with Taurine in L6 Cells
2.9. Effects of PLC Inhibitor on AMPK Phosphorylation and Myogenic Gene and Protein Expressions
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Experiments
4.3. Culture of L6 Cells
4.4. Biochemical Analysis
4.5. Histological Analysis
4.6. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR) Analysis
4.7. Western Blot Analysis
4.8. Mitochondrial DNA Analysis
4.9. Intracellular Calcium Measurements
4.10. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lubec, B.; Ya-Hua, Z.; Pertti, S.; Pentti, T.; Kitzmüller, E.; Lubec, G. Distribution and Disappearance of the Radiolabeled Carbon Derived from L-Arginine and Taurine in the Mouse. Life Sci. 1997, 60, 2373–2381. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Ju Jong, C.; Kc, R.; Azuma, J. Physiological Roles of Taurine in Heart and Muscle. J. Biomed. Sci. 2010, 17, S2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huxtable, R.J. Physiological Actions of Taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, H.; Ohnishi, N. Occurrence of Taurine in Plants. Agric. Biol. Chem. 1986, 50, 1887–1888. [Google Scholar] [CrossRef]
- Hosomi, R.; Yoshida, M.; Fukunaga, K. Seafood Consumption and Components for Health. Glob. J. Health Sci. 2012, 4, 72–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isono, C.; Maruta, H.; Ma, Y.; Ganeko, N.; Miyake, T.; Yamashita, H. Seasonal Variations in Major Components of Crassostrea Gigas from Seto Inland Sea. Fish. Sci. 2020, 86, 1087–1099. [Google Scholar] [CrossRef]
- Yang, J.Y.; Zhang, T.T.; Yu, Z.L.; Wang, C.C.; Zhao, Y.C.; Wang, Y.M.; Xue, C.H. Taurine Alleviates Trimethylamine N-Oxide-Induced Atherosclerosis by Regulating Bile Acid Metabolism in ApoE-/-Mice. J. Agric. Food Chem. 2022, 70, 5738–5747. [Google Scholar] [CrossRef]
- Wu, G. Important Roles of Dietary Taurine, Creatine, Carnosine, Anserine and 4-Hydroxyproline in Human Nutrition and Health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef] [Green Version]
- Nieminen, M.-L.; Tuomisto, L.; Solatunturi, E.; Eriksson, L.; Paasonen, M.K. Taurine in the Osmoregulation of the Brattlrbord Rat. Life Sci. 1988, 42, 2137–2143. [Google Scholar] [CrossRef]
- Spriet, L.L.; Whitfield, J. Taurine and Skeletal Muscle Function. Curr. Opin. Clin. Nutr. Metab. Care. 2015, 18, 96–101. [Google Scholar] [CrossRef]
- Seidel, U.; Huebbe, P.; Rimbach, G. Taurine: A Regulator of Cellular Redox Homeostasis and Skeletal Muscle Function. Mol. Nutr. Food Res. 2019, 63, 1–58. [Google Scholar] [CrossRef]
- Warskulat, U.; Flögel, U.; Jacoby, C.; Hartwig, H.G.; Thewissen, M.; Merx, M.W.; Molojavyi, A.; Heller-Stilb, B.; Schrader, J.; Häussinger, D. Taurine Transporter Knockout Depletes Muscle Taurine Levels and Results in Severe Skeletal Muscle Impairment but Leaves Cardiac Function Uncompromised. FASEB J. 2004, 18, 577–579. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Yoshikawa, N.; Inui, T.; Miyazaki, N.; Schaffer, S.W.; Azuma, J. Tissue Depletion of Taurine Accelerates Skeletal Muscle Senescence and Leads to Early Death in Mice. PLoS ONE 2014, 9, e107409. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, T.; Matsuzaki, Y.; Ikegami, T.; Miyakawa, S.; Doy, M.; Tanaka, N.; Bouscarel, B. Optimal and Effective Oral Dose of Taurine to Prolong Exercise Performance in Rat. Amino Acids 2004, 27, 291–298. [Google Scholar] [CrossRef]
- Pierno, S.; De Luca, A.; Camerino, C.; Huxtable, R.J.; Camerino, D.C. Chronic Administration of Taurine to Aged Rats Improves the Electrical and Contractile Properties of Skeletal Muscle Fibers1. J. Pharmacol. Exp. Ther. 1998, 286, 1183–1190. [Google Scholar]
- Ma, Y.; Maruta, H.; Sun, B.; Wang, C.; Isono, C.; Yamashita, H. Effects of Long-Term Taurine Supplementation on Age-Related Changes in Skeletal Muscle Function of Sprague–Dawley Rats. Amino Acids 2021, 53, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. Minireview: The AMP-Activated Protein Kinase Cascade: The Key Sensor of Cellular Energy Status. Endocrinology 2003, 144, 5179–5183. [Google Scholar] [CrossRef] [PubMed]
- Minokoshi, Y.; Kim, Y.-B.; Peroni, O.D.; Fryer, L.G.D.; Müller, C.; Carling, D.; Kahn, B.B. Leptin Stimulates Fatty-Acid Oxidation by Activating AMP-Activated Protein Kinase. Nature 2002, 415, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Scott, J.W.; Pan, D.A.; Hudson, E.R. Management of Cellular Energy by the AMP-Activated Protein Kinase System. FEBS Lett. 2003, 546, 113–120. [Google Scholar] [CrossRef]
- Hardie, D.G. Sensing of Energy and Nutrients by AMP-Activated Protein Kinase. Am. J. Clin. Nutr. 2011, 93, 891S–896S. [Google Scholar] [CrossRef] [Green Version]
- Yatabe, Y.; Miyakawa, S.; Miyazaki, T.; Matsuzaki, Y.; Ochiai, N. Effects of Taurine Administration in Rat Skeletal Muscles on Exercise. J. Orthop. Sci. 2003, 8, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.; Biasetti, M.; Messina, S.; Dominy, J. The Cytoprotective Role of Taurine in Exercise-Induced Muscle Injury. Amino Acids 2002, 22, 309–324. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Pierno, S.; Camerino, D.C. Taurine: The Appeal of a Safe Amino Acid for Skeletal Muscle Disorders. J. Transl. Med. 2015, 13, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjøbsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.N.; Pehmøller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in Skeletal Muscle Function and Metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef] [Green Version]
- Maruta, H.; Yoshimura, Y.; Araki, A.; Kimoto, M.; Takahashi, Y.; Yamashita, H. Activation of AMP-Activated Protein Kinase and Stimulation of Energy Metabolism by Acetic Acid in L6 Myotube Cells. PLoS ONE 2016, 11, e0158055. [Google Scholar] [CrossRef] [Green Version]
- Kanatous, S.B.; Mammen, P.P.A. Regulation of Myoglobin Expression. J. Exp. Biol. 2010, 213, 2741–2747. [Google Scholar] [CrossRef] [Green Version]
- Jäer, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-Activated Protein Kinase (AMPK) Action in Skeletal Muscle via Direct Phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [Green Version]
- Holmes, B.F.; Sparling, D.P.; Olson, A.L.; Winder, W.W.; Dohm, G.L. Regulation of Muscle GLUT4 Enhancer Factor and Myocyte Enhancer Factor 2 by AMP-Activated Protein Kinase. Am. J. Physiol.-Endocrinol. Metab. 2005, 289, 1071–1076. [Google Scholar] [CrossRef]
- Mangum, J.E.; Hardee, J.P.; Fix, D.K.; Puppa, M.J.; Elkes, J.; Altomare, D.; Bykhovskaya, Y.; Campagna, D.R.; Schmidt, P.J.; Sendamarai, A.K.; et al. Pseudouridine Synthase 1 Deficient Mice, a Model for Mitochondrial Myopathy with Sideroblastic Anemia, Exhibit Muscle Morphology and Physiology Alterations. Sci. Rep. 2016, 6, 26202 . [Google Scholar] [CrossRef] [Green Version]
- Van Der Zwaard, X.S.; De Ruiter, C.J.; Noordhof, D.A.; Sterrenburg, R.; Bloemers, F.W.; De Koning, J.J.; Jaspers, R.T.; Van Der Laarse, W.J. Maximal Oxygen Uptake Is Proportional to Muscle Fiber Oxidative Capacity, from Chronic Heart Failure Patients to Professional Cyclists. J. Appl. Physiol. 2016, 121, 636–645. [Google Scholar] [CrossRef] [Green Version]
- White, J.P.; Baltgalvis, K.A.; Puppa, M.J.; Sato, S.; Baynes, J.W.; Carson, J.A. Muscle Oxidative Capacity during IL-6-Dependent Cancer Cachexia. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 300, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huxtable, R.J.; Laird, H.E.; Lippincott, S. The Transport of Taurine in the Heart and the Rapid Depletion of Tissue Taurine Content by Guanidinoethyl Sulfonate. J. Pharmacol. Exp. Ther. 1979, 211, 465–471. [Google Scholar] [PubMed]
- Iwata, H.; Obara, T.; Kim, B.K.; Baba, A. Regulation of Taurine Transport in Rat Skeletal Muscle. J. Neurochem. 1986, 47, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.B.; Hu, X.; Zhuang, X.D.; Liao, L.Z.; Li, W.D. GYY4137, a Novel Hydrogen Sulfide-Releasing Molecule, Likely Protects against High Glucose-Induced Cytotoxicity by Activation of the AMPK/MTOR Signal Pathway in H9c2 Cells. Mol. Cell. Biochem. 2014, 389, 249–256. [Google Scholar] [CrossRef]
- Abbott, M.J.; Edelman, A.M.; Turcotte, L.P. CaMKK Is an Upstream Signal of AMP-Activated Protein Kinase in Regulation of Substrate Metabolism in Contracting Skeletal Muscle. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, 1724–1733. [Google Scholar] [CrossRef] [Green Version]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M. Adiponectin and AdipoR1 Regulate PGC-1alpha and Mitochondria by Ca(2+) and AMPK/SIRT1. Nature 2010, 464, 1313–1319. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kawaguchi, Y.; Fujimoto, T.; Kanayama, N.; Magari, M.; Tokumitsu, H. Differential AMP-Activated Protein Kinase (AMPK) Recognition Mechanism of Ca2+/Calmodulin-Dependent Protein Kinase Kinase Isoforms. J. Biol. Chem. 2016, 291, 13802–13808. [Google Scholar] [CrossRef] [Green Version]
- Vines, C.M. Phospholipase C. In Advances in Experimental Medicine and Biology; Springer New York LLC: New York, NY, USA, 2020; Volume 1131, pp. 215–242. [Google Scholar] [CrossRef]
- Maruta, H.; Yamashita, H. Acetic Acid Stimulates G-Protein-Coupled Receptor GPR43 and Induces Intracellular Calcium Influx in L6 Myotube Cells. PLoS ONE 2020, 15, e0239428. [Google Scholar] [CrossRef]
- Sved, D.W.; Godsey, J.L.; Ledyard, S.L.; Mahoney, A.P.; Stetson, P.L.; Ho, S.; Myers, N.R.; Resnis, P.; Renwick, A.G. Absorption, Tissue Distribution, Metabolism and Elimination of Taurine given Orally to Rats. Amino Acids 2007, 32, 459–466. [Google Scholar] [CrossRef]
- Hardie, D.G.; Sakamoto, K. AMPK: A Key Sensor of Fuel and Energy Status in Skeletal Muscle. Physiology 2006, 21, 48–60. [Google Scholar] [CrossRef]
- Lantier, L.; Fentz, J.; Mounier, R.; Leclerc, J.; Treebak, J.T.; Pehmøller, C.; Sanz, N.; Sakakibara, I.; Saint-Amand, E.; Rimbaud, S.; et al. AMPK Controls Exercise Endurance, Mitochondrial Oxidative Capacity, and Skeletal Muscle Integrity. FASEB J. 2014, 28, 3211–3224. [Google Scholar] [CrossRef] [PubMed]
- Winder, W.W.; Holmes, B.F.; Rubink, D.S.; Jensen, E.B.; Chen, M.; Holloszy, J.O. Activation of AMP-Activated Protein Kinase Increases Mitochondrial Enzymes in Skeletal Muscle. J. Appl. Physiol. 2000, 88, 2219–2226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thai, M.V.; Guruswamy, S.; Cao, K.T.; Pessin, J.E.; Olson, A.L. Myocyte Enhancer Factor 2 (MEF2)-Binding Site Is Required for GLUT4 Gene Expression in Transgenic Mice: Regulation of MEF2 DNA Binding Activity in Insulin-Deficient Diabetes. J. Biol. Chem. 1998, 273, 14285–14292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Czech, M.P. The GLUT4 Glucose Transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N. Transcriptional Co-Activator PGC-1 Alpha Drives the Formation of Slow-Twitch Muscle Fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef]
- Pilegaard, H.; Saltin, B.; Neufer, D.P. Exercise Induces Transient Transcriptional Activation of the PGC-1α Gene in Human Skeletal Muscle. J. Physiol. 2003, 546, 851–858. [Google Scholar] [CrossRef]
- Irrcher, I.; Ljubicic, V.; Kirwan, A.F.; Hood, D.A. AMP-Activated Protein Kinase-Regulated Activation of the PGC-1α Promoter in Skeletal Muscle Cells. PLoS ONE 2008, 3, e3614. [Google Scholar] [CrossRef] [Green Version]
- Terada, S.; Goto, M.; Kato, M.; Kawanaka, K.; Shimokawa, T.; Tabata, I. Effects of Low-Intensity Prolonged Exercise on PGC-1 MRNA Expression in Rat Epitrochlearis Muscle. Biochem. Biophys. Res. Commun. 2002, 296, 350–354. [Google Scholar] [CrossRef]
- Cooper, M.P.; Uldry, M.; Kajimura, S.; Arany, Z.; Spiegelman, B.M. Modulation of PGC-1 Coactivator Pathways in Brown Fat Differentiation through LRP130. J. Biol. Chem. 2008, 283, 31960–31967. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Jiang, F.; Huang, B.; Zheng, W.; Jiang, Y.; Cai, G.; Liu, D.; Hu, C.Y.; Wang, C. Dihydromyricetin Ameliorates Inflammation-Induced Insulin Resistance via Phospholipase C-CaMKK-AMPK Signal Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Maruta, H.; Abe, R.; Yamashita, H. Effect of Long-Term Supplementation with Acetic Acid on the Skeletal Muscle of Aging Sprague Dawley Rats. Int. J. Mol. Sci. 2022, 23, 4691. [Google Scholar] [CrossRef] [PubMed]
- Miletta, M.C.; Petkovic, V.; Eblé, A.; Ammann, R.A.; Flück, C.E.; Mullis, P.E. Butyrate Increases Intracellular Calcium Levels and Enhances Growth Hormone Release from Rat Anterior Pituitary Cells via the G-Protein-Coupled Receptors GPR41 and 43. PLoS ONE 2014, 9, e107388. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Gene | Direction | Primer Sequence |
|---|---|---|
| β-actin (Actb) | Forward | 5′-GGAGATTACTGCCCTGGCTCCTA-3′ |
| Reverse | 5′-GACTCATCGTACTCCTGCTTGCTG-3′ | |
| MEF2A (Mef2a) | Forward | 5′-ATGAGAGGAACCGACAGGTG-3′ |
| Reverse | 5′-TATCCGAGTTCGTCCTGCTT-3′ | |
| PGC-1α (Ppargc1a) | Forward | 5′-GACCCCAGAGTCACCAAATGA-3′ |
| Reverse | 5′-GGCCTGCAGTTCCAGAGAGT-3′ | |
| Succinate dehydrogenase (Sdha) | Forward | 5′-TGGGGCGACTCGTGGCTTTC-3′ |
| Reverse | 5′-CCCCGCCTGCACCTACAACC-3′ | |
| Cytochrome C, somatic (Cycs) | Forward | 5′-AGCGGGACGTCTCCCTAAGA-3′ |
| Reverse | 5′-CTTCCGCCCAAACAGACCA-3′ | |
| Myoglobin (Mb) | Forward | 5′-CTAACAGCCGGCCTACACTC-3′ |
| Reverse | 5′-CGTGCTTCTTCAGGTCCTCT-3′ | |
| GLUT4 (Slc2a4) | Forward | 5′-GGGCGATTTCTCCCACATAC-3′ |
| Reverse | 5′-CTCATGGGCCTAGCCAATG-3′ | |
| TauT (Slc6a6) | Forward | 5′-CAGTGCCACAGCCTCTTCAG-3′ |
| Reverse | 5′-CTTGCTGGACCACTTCTCCC-3′ | |
| Mitochondrial NADH dehydrogenase 1 (Mt-Nd1) | Forward | 5′-CTCCCTATTCGGAGCCCTAC-3′ |
| Reverse | 5′-ATTTGTTTCTGCTAGGGTTG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.; Maruta, H.; Ma, Y.; Yamashita, H. Taurine Stimulates AMP-Activated Protein Kinase and Modulates the Skeletal Muscle Functions in Rats via the Induction of Intracellular Calcium Influx. Int. J. Mol. Sci. 2023, 24, 4125. https://doi.org/10.3390/ijms24044125
Sun B, Maruta H, Ma Y, Yamashita H. Taurine Stimulates AMP-Activated Protein Kinase and Modulates the Skeletal Muscle Functions in Rats via the Induction of Intracellular Calcium Influx. International Journal of Molecular Sciences. 2023; 24(4):4125. https://doi.org/10.3390/ijms24044125
Chicago/Turabian StyleSun, Baojun, Hitomi Maruta, Yun Ma, and Hiromi Yamashita. 2023. "Taurine Stimulates AMP-Activated Protein Kinase and Modulates the Skeletal Muscle Functions in Rats via the Induction of Intracellular Calcium Influx" International Journal of Molecular Sciences 24, no. 4: 4125. https://doi.org/10.3390/ijms24044125