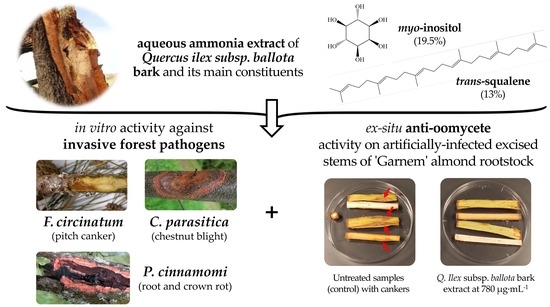
Holm Oak (Quercus ilex subsp. ballota (Desf.) Samp.) Bark Aqueous Ammonia Extract for the Control of Invasive Forest Pathogens
Abstract
:1. Introduction
2. Results
2.1. Phytochemicals Identified by GC–MS
2.2. In Vitro Antimicrobial Activity
2.3. Protection of Excised Stems against P. cinnamomi
3. Discussion
3.1. On the Phytochemical Composition
3.2. Antimicrobial Activity Comparison
3.3. Comparison of Efficacy vs. Other Natural Compounds
3.4. Comparison with a Conventional Fungicide
3.5. Mode of Action
4. Material and Methods
4.1. Reagents
4.2. Phytopathogen Isolates
4.3. Plant Material
4.4. Preparation of the Extract
4.5. Characterization of the Extract
4.6. In Vitro Antimicrobial Activity Assessment
4.7. Protection Tests on Artificially Inoculated Excised Stems
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sousa, V.; Ferreira, J.P.A.; Miranda, I.; Quilhó, T.; Pereira, H. Quercus rotundifolia bark as a source of polar extracts: Structural and chemical characterization. Forests 2021, 12, 1160. [Google Scholar] [CrossRef]
- Montserrat-Martí, G.; Camarero, J.J.; Palacio, S.; Pérez-Rontomé, C.; Milla, R.; Albuixech, J.; Maestro, M. Summer-drought constrains the phenology and growth of two coexisting Mediterranean oaks with contrasting leaf habit: Implications for their persistence and reproduction. Trees 2009, 23, 787–799. [Google Scholar] [CrossRef] [Green Version]
- Ribechini, E.; Mangani, F.; Colombini, M.P. Chemical investigation of barks from broad-leaved tree species using EGA-MS and GC/MS. J. Anal. Appl. Pyrolysis 2015, 114, 235–242. [Google Scholar] [CrossRef]
- Meziti, H.; Bouriche, H.; Kada, S.; Demirtas, I.; Kizil, M.; Senator, A.; Garrido, G. Phytochemical analysis, and antioxidant, anti-hemolytic and genoprotective effects of Quercus ilex L. and Pinus halepensis Mill. methanolic extracts. J Pharm. Pharmacogn. Res. 2019, 7, 260–272. [Google Scholar]
- Karioti, A.; Bilia, A.R.; Skaltsa, H. Quercus ilex L.: A rich source of polyacylated flavonoid glucosides. Food Chem. 2010, 123, 131–142. [Google Scholar] [CrossRef]
- Hadidi, L.; Babou, L.; Zaidi, F.; Valentão, P.; Andrade, P.B.; Grosso, C. Quercus ilex L.: How season, plant organ and extraction procedure can influence chemistry and bioactivities. Chem. Biodivers. 2017, 14, e1600187. [Google Scholar] [CrossRef]
- Burlacu, E.; Nisca, A.; Tanase, C. A comprehensive review of phytochemistry and biological activities of Quercus species. Forests 2020, 11, 904. [Google Scholar] [CrossRef]
- Dallali, S.; Zouaouia, R.; Dallalic, D.; Jdidia, S.; Toumia, L. Determination of some biochemical parameters from leaves of Quercus ilex L.(Fagaceae), collected in Djabel Zagouan (Tunisia). Arab. J. Med. Aromat. Plants 2021, 7, 1–28. [Google Scholar]
- Morales, D. Oak trees (Quercus spp.) as a source of extracts with biological activities: A narrative review. Trends Food Sci. Technol. 2021, 109, 116–125. [Google Scholar] [CrossRef]
- Berahou, A.; Auhmani, A.; Fdil, N.; Benharref, A.; Jana, M.; Gadhi, C.A. Antibacterial activity of Quercus ilex bark’s extracts. J. Ethnopharmacol. 2007, 112, 426–429. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef]
- Du, B.; Zhang, Z.; Liu, W.; Ye, Y.; Lu, T.; Zhou, Z.; Li, Y.; Fu, Z.; Qian, H. Acute toxicity of the fungicide azoxystrobin on the diatom Phaeodactylum tricornutum. Ecotoxicol. Environ. Saf. 2019, 168, 72–79. [Google Scholar] [CrossRef]
- Garaniya, N.; Bapodra, A. Ethno botanical and phytopharmacological potential of Abrus precatorius L.: A review. Asian Pac. J. Trop. Biomed. 2014, 4, S27–S34. [Google Scholar] [CrossRef] [Green Version]
- Yong, J.W.; Ge, L.; Ng, Y.F.; Tan, S.N. The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef] [Green Version]
- Nguyen Thi, T.N.; Kamenarska, Z.; Handjieva, N.; Bankova, V.; Popov, S. GC-MS study on polar constituents of Crinum latifolium. Comptes Rendus L’academie Bulg. Des Sci. 2001, 54, 8–41. [Google Scholar]
- Lakshmi, V.; Gupta, P.; Tiwari, P.; Srivastava, A.K. Antihyperglycemic activity of Rhizophora apiculata Bl. in rats. Nat. Prod. Res. 2006, 20, 1295–1299. [Google Scholar] [CrossRef]
- Vivekanandadasan, V.; Rajangam, U. GC-MS analysis of leaf, fruits and latex of Croton bonplandianum Baill. Int. J. Biochem. Res. Rev. 2015, 5, 187–197. [Google Scholar]
- Suman, T.Y.; Elumalai, D.; Kaleena, P.K.; Rajasree, S.R.R. GC–MS analysis of bioactive components and synthesis of silver nanoparticle using Ammannia baccifera aerial extract and its larvicidal activity against malaria and filariasis vectors. Ind. Crops Prod. 2013, 47, 239–245. [Google Scholar] [CrossRef]
- Stevenson, D.G.; Eller, F.J.; Wang, L.; Jane, J.-L.; Wang, T.; Inglett, G.E. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J. Agric. Food. Chem. 2007, 55, 4005–4013. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.H.; Chen, F.L.; Tan, X.M. Gas chromatographic-mass spectrometric analysis of essential oil of Jasminum officinale L. var grandiflorum flower. Trop. J. Pharm. Res. 2015, 14, 149–152. [Google Scholar] [CrossRef] [Green Version]
- Anandan, A.; Eswaran, R.; Doss, A.; Sangeetha, G.; Anand, S. Chemical compounds investigation of Lucas aspera leaves-a potential folklore medicinal plant. Asian J. Pharm. Clin. Res. 2012, 5, 86–88. [Google Scholar]
- Nagella, P.; Ahmad, A.; Kim, S.-J.; Chung, I.-M. Chemical composition, antioxidant activity and larvicidal effects of essential oil from leaves of Apium graveolens. Immunopharmacol. Immunotoxicol. 2011, 34, 205–209. [Google Scholar] [CrossRef]
- Boy, F.R.; Casquete, R.; Martínez, A.; Córdoba, M.d.G.; Ruíz-Moyano, S.; Benito, M.J. Antioxidant, antihypertensive and antimicrobial properties of phenolic compounds obtained from native plants by different extraction methods. Int. J. Environ. Res. Public Health 2021, 18, 2475. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Gómez-García, R.; Carrasco, E.; Bascón-Villegas, I.; Rodríguez, A.; Pintado, M. Quercus ilex leaf as a functional ingredient: Polyphenolic profile and antioxidant activity throughout simulated gastrointestinal digestion and antimicrobial activity. J. Funct. Foods 2022, 91, 105025. [Google Scholar] [CrossRef]
- Güllüce, M.; Adıgüzel, A.; Öğütçü, H.; Şengül, M.; Karaman, İ.; Şahin, F. Antimicrobial effects of Quercus ilex L. extract. Phytother. Res. 2004, 18, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Merghache, D.; Boucherit-Otmani, Z.; El Haci, I.A.; Chikhi, I.; Boucherit, K. Inhibitory effect of Quercus ilex wood ash on the growth of pathogenic microorganisms. Phytothérapie 2019, 16, S269–S272. [Google Scholar] [CrossRef]
- Bakour, M.; Laaroussi, H.; Ousaaid, D.; Oumokhtar, B.; Lyoussi, B.; Romeo, F.V. Antioxidant and antibacterial effects of pollen extracts on human multidrug-resistant pathogenic bacteria. J. Food Qual. 2021, 2021, 5560182. [Google Scholar] [CrossRef]
- Akroum, S. Antifungal activity of acetone extracts from Punica granatum L., Quercus suber L. and Vicia faba L. J. Mycol. Médicale 2017, 27, 83–89. [Google Scholar] [CrossRef]
- O. Elansary, H.; Szopa, A.; Kubica, P.; Ekiert, H.; A. Mattar, M.; Al-Yafrasi, M.A.; El-Ansary, D.O.; Zin El-Abedin, T.K.; Yessoufou, K. Polyphenol profile and pharmaceutical potential of Quercus spp. bark extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef] [Green Version]
- Dania, V.O.; Fadina, O.O.; Ayodele, M.; Kumar, P.L. Efficacy of Oryza sativa husk and Quercus phillyraeoides extracts for the in vitro and in vivo control of fungal rot disease of white yam (Dioscorea rotundata Poir). SpringerPlus 2014, 3, 711. [Google Scholar] [CrossRef] [Green Version]
- Gul, F.; Khan, K.M.; Adhikari, A.; Zafar, S.; Akram, M.; Khan, H.; Saeed, M. Antimicrobial and antioxidant activities of a new metabolite from Quercus incana. Nat. Prod. Res. 2016, 31, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Iturritxa, E.; Trask, T.; Mesanza, N.; Raposo, R.; Elvira-Recuenco, M.; Patten, C. Biocontrol of Fusarium circinatum infection of young Pinus radiata trees. Forests 2017, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Seseni, L.; Regnier, T.; Roux-van der Merwe, M.P.; Mogale, E.; Badenhorst, J. Control of Fusarium spp. causing damping-off of pine seedlings by means of selected essential oils. Ind. Crops Prod. 2015, 76, 329–332. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, J.; Lee, S.-G.; Oh, E.; Shin, S.-C.; Park, I.-K. Effects of plant essential oils and components from Oriental sweetgum (Liquidambar orientalis) on growth and morphogenesis of three phytopathogenic fungi. Pestic. Biochem. Physiol. 2009, 93, 138–143. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, J.; Shin, S.-C.; Lee, S.-G.; Park, I.-K. Antifungal activity of Myrtaceae essential oils and their components against three phytopathogenic fungi. Flavour Fragr. J. 2008, 23, 23–28. [Google Scholar] [CrossRef]
- Lukovic, J.; Potocnik, I.; Rekanovic, E.; Milijasevic-Marcic, S.; Todorovic, B.; Kostic, M.; Radulovic, Z. Toxicity of plant essential oils to Cryphonectria parasitica (Murr.) Barr, the causal agent of chestnut blight. Pestic. Fitomedicina 2019, 34, 89–96. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.S.; Lee, S.G.; Shin, S.C.; Park, I.K. Fumigant antifungal activity of plant essential oils and components from West Indian bay (Pimenta racemosa) and thyme (Thymus vulgaris) oils against two phytopathogenic fungi. Flavour Fragr. J. 2008, 23, 272–277. [Google Scholar] [CrossRef]
- Castillo-Reyes, F.; Hernández-Castillo, F.D.; Clemente-Constantino, J.A.; Gallegos-Morales, G.; Rodríguez-Herrera, R.; Noé-Aguilar, C. In vitro antifungal activity of polyphenols-rich plant extracts against Phytophthora cinnamomi Rands. Afr. J. Agric. Res. 2015, 10, 4554–4560. [Google Scholar] [CrossRef] [Green Version]
- Giamperi, L.; Fraternale, D.; Ricci, D. The in vitro action of essential oils on different organisms. J. Essent. Oil Res. 2011, 14, 312–318. [Google Scholar] [CrossRef]
- Carvajal, M.A.; Vergara, A.P.; Santander, R.; Osorio, M.E. Chemical composition and anti-phytopathogenic activity of the essential oil of Beilschmiedia miersii. Nat. Prod. Commun. 2016, 11, 1367–1372. [Google Scholar] [CrossRef] [Green Version]
- Moiteiro, C.; Esteves, T.; Ramalho, L.; Rojas, R.; Alvarez, S.; Zacchino, S.; Bragança, H. Essential oil characterization of two Azorean Cryptomeria japonica populations and their biological evaluations. Nat. Prod. Commun. 2013, 8, 1785–1790. [Google Scholar] [CrossRef] [Green Version]
- Martins, J.; Batista, T.; Pinto, G.; Canhoto, J. Seasonal variation of phenolic compounds in strawberry tree (Arbutus unedo L.) leaves and inhibitory potential on Phytophthora cinnamomi. Trees 2021, 35, 1571–1586. [Google Scholar] [CrossRef]
- Leadbeater, A.J. Plant Health Management: Fungicides and Antibiotics. In Encyclopedia of Agriculture and Food Systems; Van Alfen, N.K., Ed.; Academic Press: Amsterdam, The Netherlands, 2014; pp. 408–424. [Google Scholar] [CrossRef]
- Benalcázar Villalba, S.M. Sensibilidad In Vitro de Fusarium spp. Asociado a Muerte de Plantulas de Pinus Radiata y Pinus Patula a Fungicidas de Diferente modo de Acción; Polytechnic School of Chimborazo: Riobamba, Ecuador, 2021. [Google Scholar]
- González-Varela, G.; González, A.J. In vitro sensitivity of Cryphonectria parasitica to six agrochemicals. Australas. Plant Dis. Notes 2007, 2, 109–110. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.-l.; Chen, H.; Zhong, K.-K.; Li, L.; Kong, X.-H. Myo-inositol as an adjuvant to florfenicol against Aeromonas hydrophila infection in common carp Cyprinus carpio. FEMS Microbiol. Lett. 2018, 365, fny212. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Al-Suod, H.; Ligor, M.; Ligor, T.; Krakowska, A.; Górecki, R.; Buszewski, B. Simultaneous determination of cyclitols and sugars following a comprehensive investigation of 40 plants. Food Anal. Methods 2019, 12, 1466–1478. [Google Scholar] [CrossRef] [Green Version]
- Elsherbiny, E.A.; Safwat, N.A.; Elaasser, M.M. Fungitoxicity of organic extracts of Ocimum basilicum on growth and morphogenesis of Bipolaris species (teleomorph Cochliobolus). J. Appl. Microbiol. 2017, 123, 841–852. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Elakkad, H.A.; El-Tahan, A.M.; Alshahrani, O.A.; Alshilawi, M.S.; El-Sayed, H.; Amin, S.A.; Ahmed, A.I. Flavoring and extending the shelf life of cucumber juice with aroma compounds-rich herbal extracts at 4 °C through controlling chemical and microbial fluctuations. Saudi J. Biol. Sci. 2022, 29, 346–354. [Google Scholar] [CrossRef]
- Shafiei, S.N.S.; Ahmad, K.; Ikhsan, N.; Ismail, S.I.; Sijam, K. Antibacterial activity of Acacia spp. leaves extracts against Xanthomonas oryzae pv. oryzae and screening for active phytochemical contents. IOSR J. Agric. Vet. Sci. 2017, 10, 49–60. [Google Scholar]
- Langa-Lomba, N.; Sánchez-Hernández, E.; Buzón-Durán, L.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Activity of anthracenediones and flavoring phenols in hydromethanolic extracts of Rubia tinctorum against grapevine phytopathogenic fungi. Plants 2021, 10, 1527. [Google Scholar] [CrossRef]
- Ryder, N.S. Terbinafine: Mode of action and properties of the squalene epoxidase inhibition. Br. J. Dermatol. 1992, 126, 2–7. [Google Scholar] [CrossRef]
- Elewski, B.E. Mechanisms of action of systemic antifungal agents. J. Am. Acad. Dermatol. 1993, 28, S28–S34. [Google Scholar] [CrossRef]
- Ma, Q.-Z.; Wu, F.-J.; Liu, Q.-M.; Li, K.-F.; Xie, C.-P. Analysis on volatile organic compounds of Artocarpus lingnanensis for urban environmental management. In Proceedings of the 2010 International Conference on Management and Service Science, Wuhan, China, 24–26 August 2010; pp. 1–4. [Google Scholar]
- Antoci, V.; Oniciuc, L.; Amariucai-Mantu, D.; Moldoveanu, C.; Mangalagiu, V.; Amarandei, A.M.; Lungu, C.N.; Dunca, S.; Mangalagiu, I.I.; Zbancioc, G. Benzoquinoline derivatives: A straightforward and efficient route to antibacterial and antifungal agents. Pharmaceuticals 2021, 14, 335. [Google Scholar] [CrossRef]
- Radoykova, T.; Stanulov, K.; Nenkova, S. Lignin-derived methoxyphenols as antioxidant additives for gasoline. Oxid. Commun. 2011, 34, 463. [Google Scholar]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Vernon, D.M.; Ostrem, J.A.; Bohnert, H.J. Stress perception and response in a facultative halophyte: The regulation of salinity-induced genes in Mesembryanthemum crystallinum. Plant Cell Environ. 1993, 16, 437–444. [Google Scholar] [CrossRef]
- Naika, R.; Pavani, P. Evaluation of antibacterial activity and GCMS analysis of Zanthoxylum ovalifolium fruit extracts. J. Pharm. Res. Int. 2021, 33, 7–17. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Martín-Ramos, P.; Martín-Gil, J.; Santiago-Aliste, A.; Hernández-Navarro, S.; Oliveira, R.; González-García, V. Bark extract of Uncaria tomentosa L. for the control of strawberry phytopathogens. Horticulturae 2022, 8, 672. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matheron, M.E. Seasonal variation in susceptibility of Juglans hindsii and Paradox rootstocks of English walnut trees to Phytophthora citricola. Phytopathology 1985, 75, 970–972. [Google Scholar] [CrossRef]
- Si, Y.; Haxim, Y.; Wang, L. Optimum sterilization method for in vitro cultivation of dimorphic seeds of the succulent halophyte Suaeda aralocaspica. Horticulturae 2022, 8, 289. [Google Scholar] [CrossRef]




| Peak | RT (min) | Area (%) | Assignment |
|---|---|---|---|
| 1 | 4.3897 | 4.3045 | oxime-, methoxy-phenyl-_ |
| 2 | 4.6983 | 4.5463 | 1-pentanol |
| 3 | 4.7695 | 2.7271 | 2-cyclopent-2-en-1-one, 2-hydroxy- |
| 4 | 5.7607 | 2.2880 | succindialdehyde |
| 5 | 5.8379 | 3.3537 | 2-hydroxy-γ-butyrolactone |
| 6 | 7.2861 | 1.2101 | 2-methoxy-phenol |
| 7 | 7.3573 | 1.4969 | pentanal |
| 8 | 8.9064 | 4.4657 | catechol |
| 9 | 9.0489 | 2.3118 | 1H-tetrazole, 5-(trifluoromethyl)- |
| 10 | 9.8620 | 1.1263 | pyridine, 4-nitro-, 1-oxide |
| 11 | 10.3843 | 0.5025 | 1H-imidazole-4-methanol, 5-methyl- |
| 12 | 11.0491 | 1.2235 | 2,6-dimethoxy-phenol |
| 13 | 11.6664 | 1.1952 | 3-octyne |
| 14 | 12.2183 | 11.4443 | 1-butanol, 4-butoxy- |
| 15 | 12.5804 | 1.0696 | 2-trifluoroacetoxytridecane |
| 16 | 14.2245 | 1.7480 | 3,4,5-trimethoxy-phenol |
| 17 | 14.8833 | 6.9425 | allo-inositol |
| 18 | 14.9961 | 3.8720 | inositol, 1-deoxy- |
| 19 | 15.0258 | 0.9823 | inositol, 1-deoxy- |
| 20 | 15.0910 | 3.6660 | d-lyxose |
| 21 | 15.1563 | 2.8651 | l-lyxose |
| 22 | 15.2691 | 6.2190 | d-gulopyranose |
| 23 | 15.3225 | 3.3908 | d-gulopyranose |
| 24 | 15.3463 | 7.6969 | myo-inositol |
| 25 | 17.9103 | 1.2863 | n-nexadecanoic acid |
| 26 | 25.0920 | 12.9624 | supraene (or trans-squalene) |
| 27 | 26.6352 | 0.9232 | benzo[H]quinoline, 2,4-dimethyl- |
| 28 | 28.8194 | 1.3713 | benzo[H]quinoline, 2,4-dimethyl- |
| 29 | 29.5494 | 2.8086 | benzo[H]quinoline, 2,4-dimethyl- |
| Product | Effective Concentration | F. circinatum | C. parasitica | P. cinnamomi |
|---|---|---|---|---|
| Q. ilex subsp. ballota bark extract | EC50 | 92.1 | 142.3 | 63.4 |
| EC90 | 322.4 | 294.9 | 75.2 | |
| myo-inositol | EC50 | 375.9 | 171.8 | 174.9 |
| EC90 | 710.2 | 472.6 | 321.5 | |
| trans-squalene | EC50 | 106.4 | 59.0 | 38.2 |
| EC90 | 173.6 | 135.2 | 87.8 |
| Pathogen | Source | Natural Product | Inhibitory Value | Ref. |
|---|---|---|---|---|
| F. circinatum | Aqueous ammonia bark extract (1:1) | Quercus ilex subsp. ballota | MIC = 375 µg·mL−1 | This work |
| Commercial EOs | Artemisa arborescens EO | n.a. | [34] | |
| Anthemis nobilis EO | n.a. | |||
| Coriandrum sativum EO | MIC > 28 µg·mL−1 air | |||
| Cyperus scariosus EO | MIC > 28 µg·mL−1 air | |||
| Commiphora myrrha EO | MIC > 28 µg·mL−1 air | |||
| Pastinaca sativa EO | MIC > 28 µg·mL−1 air | |||
| Pogostemon patchouli EO | MIC > 28 µg·mL−1 air | |||
| Miroxylon balsamum EO | MIC > 28 µg·mL−1 air | |||
| Salvia stenophylla EO | n.a. | |||
| Santalum album EO | n.a. | |||
| Santolina chamaecyparissus EO | n.a. | |||
| Nardostachys sinensis EO | n.a. | |||
| Liquidambar orientalis EO | MIC > 28 µg·mL−1 air | |||
| Valeriana wallichii EO | MIC > 28 µg·mL−1 air | |||
| Lippia javanica EO | n.a | |||
| Leptospermum scoparium EO | MIC > 28 µg·mL−1 air | |||
| Juniperus mexicana EO | n.a | |||
| Daucus carota EO | MIC > 28 µg·mL−1 air | |||
| Calitis intratropica EO | MIC > 28 µg·mL−1 air | |||
| Commercial EOs | Eucalyptus citriodora EO | MIC > 28 µg·mL−1 air | [35] | |
| Melaleuca quinquenervia EO | MIC > 28 µg·mL−1 air | |||
| L. petersonii EO | MIC > 28 µg·mL−1 air | |||
| Foliage, wood, and bark | Cryptomeria japonica EO | n.a. | [41] | |
| Commercial EOs | Syzygium aromaticum EO | MIC = 400–500 µL·L−1 | [33] | |
| Cymbopogon citratus EO | MIC = 400–700 µL·L−1 | |||
| Thymus vulgaris EO | MIC = 500 µL·L−1 | |||
| Pelargonium graveolens EO | MIC = 900–1000 µL·L−1 | |||
| n.e. | Cinnamomum verum EO | MIC = 10% v/v | [32] | |
| Foeniculum vulgare EO | MIC = 50% v/v | |||
| S. aromaticum EO | MIC = 15% v/v | |||
| C. parasitica | Aqueous ammonia bark extract (1:1) | Q. ilex subsp. ballota | MIC = 375 µg·mL−1 | This work |
| Commercial EOs | A. arborescens EO | MIC > 28 µg·mL−1 air | [34] | |
| A. nobilis EO | n.a | |||
| C. sativum EO | n.a | |||
| C. scariosus EO | MIC > 28 µg·mL−1 air | |||
| C. myrrha EO | n.a | |||
| P. sativa EO | MIC > 28 µg·mL−1 air | |||
| P. patchouli EO | MIC > 28 µg·mL−1 air | |||
| M. balsamum EO | MIC > 28 µg·mL−1 air | |||
| S. stenophylla EO | MIC > 28 µg·mL−1 air | |||
| S. album EO | MIC > 28 µg·mL−1 air | |||
| S. chamaecyparissus EO | MIC > 28 µg·mL−1 air | |||
| N. sinensis EO | MIC > 28 µg·mL−1 air | |||
| L. orientalis EO | MIC > 28 µg·mL−1 air | |||
| V. wallichii EO | MIC > 28 µg·mL−1 air | |||
| L. javanica EO | MIC > 28 µg·mL−1 air | |||
| L. scoparium EO | n.a | |||
| J. mexicana EO | n.a | |||
| D. carota EO | n.a | |||
| C. intratropica EO | n.a | |||
| Commercial EOs | E. citriodora EO | MIC > 28 µg·mL−1 air | [35] | |
| M. quinquenervia EO | MIC > 28 µg·mL−1 air | |||
| L. petersonii EO | MIC > 28 µg·mL−1 air | |||
| Foliage, wood, and bark | C. japonica EO | n.a. | [41] | |
| n.e. | Illicum verum EO | MIC > 0.32 µg·mL−1 air | [36] | |
| J. oxycedrus EO | MIC = 0.08–0.16 µg·mL−1 air | |||
| E. globulus EO | MIC = 0.08–0-16 µg·mL−1 air | |||
| Lavandula angustifolia EO | MIC > 0.32 µg·mL−1 air | |||
| Citrus limon EO | MIC > 0.32 µg·mL−1 air | |||
| C. flexuosus EO | MIC > 0.32 µg·mL−1 air | |||
| Mentha piperita EO | MIC = 0.02 µg·mL−1 air | |||
| Origanum vulgare EO | MIC = 0.16–0.32 µg·mL−1 air | |||
| Pinus nigra EO | MIC = 0.02 µg·mL−1 air | |||
| P. pinaster EO | MIC = 0.16–0.32 µg·mL−1 air | |||
| P. silvestris EO | MIC = 0.32 µg·mL−1 air | |||
| Rosmarinus officinalis EO | MIC ≥ 0.32 µg·mL−1 air | |||
| S. officinalis EO | MIC = 0.04 µg·mL−1 air | |||
| Abies alba EO | MIC = 0.02 µg·mL−1 air | |||
| Gaultheria procumbens EO | MIC > 0.32 µg·mL−1 air | |||
| Commercial EOs | Pimenta racemosa EO | MIC > 28 µg·mL−1 air | [37] | |
| J. oxycedrus EO | MIC > 28 µg·mL−1 air | |||
| C. nardus EO | MIC > 28 µg·mL−1 air | |||
| P. graveolens EO | MIC > 28 µg·mL−1 air | |||
| Cuminum cyminum EO | MIC > 28 µg·mL−1 air | |||
| Myristica fragrans EO | MIC > 28 µg·mL−1 air | |||
| C. martini EO | MIC > 28 µg·mL−1 air | |||
| M. pulegium EO | MIC > 28 µg·mL−1 air | |||
| M. spicata EO | MIC > 28 µg·mL−1 air | |||
| T. vulgaris EO | MIC = 14 µg·mL−1 air | |||
| P. cinnamomi | Aqueous ammonia bark extract (1:1) | Q. ilex subsp. ballota | MIC = 78.12 µg·mL−1 | This work |
| Water, ethanol (70%), lanolin (10%), or cocoa butter (10%) | L. tridentata PE | MIC90 = 11.2−7213 µg·mL−1 | [38] | |
| F. cernua PE | MIC90 = 23.6−619 µg·mL−1 | |||
| A. lechuguilla PE | MIC90 = 58.5−327 µg·mL−1 | |||
| Opuntia ficus-indica PE | MIC90 = 3595−409, 181 µg·mL−1 | |||
| L. graveolens PE | MIC90 = 4825−n.a. µg·mL−1 | |||
| Carya illinoensis PE | n.a. | |||
| Yucca filifera PE | n.a. | |||
| n.e. | S. officinalis EO | MIC > 1600 µg·mL−1 | [39] | |
| S. rosmarinus EO | MIC > 1600 µg·mL−1 | |||
| O. vulgare EO | MIC > 200 µg·mL−1 | |||
| Laurus nobilis EO | MIC > 1600 µg·mL−1 | |||
| C. sativum EO | MIC = 800 µg·mL−1 | |||
| T. vulgaris EO | MIC = 200 µg·mL−1 | |||
| M. piperita EO | MIC = 800 µg·mL−1 | |||
| L. intermedia EO | MIC = 1600 µg·mL−1 | |||
| Aerial parts | Beilschmiedia miersii EO | MIC = 300 µg·mL−1 | [40] | |
| Leaf methanol extract (1:5) | Arbutus unedo PE | MIC = 5990 µg·mL−1 | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Hernández, E.; Balduque-Gil, J.; Barriuso-Vargas, J.J.; Casanova-Gascón, J.; González-García, V.; Cuchí-Oterino, J.A.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Holm Oak (Quercus ilex subsp. ballota (Desf.) Samp.) Bark Aqueous Ammonia Extract for the Control of Invasive Forest Pathogens. Int. J. Mol. Sci. 2022, 23, 11882. https://doi.org/10.3390/ijms231911882
Sánchez-Hernández E, Balduque-Gil J, Barriuso-Vargas JJ, Casanova-Gascón J, González-García V, Cuchí-Oterino JA, Lorenzo-Vidal B, Martín-Gil J, Martín-Ramos P. Holm Oak (Quercus ilex subsp. ballota (Desf.) Samp.) Bark Aqueous Ammonia Extract for the Control of Invasive Forest Pathogens. International Journal of Molecular Sciences. 2022; 23(19):11882. https://doi.org/10.3390/ijms231911882
Chicago/Turabian StyleSánchez-Hernández, Eva, Joaquín Balduque-Gil, Juan J. Barriuso-Vargas, José Casanova-Gascón, Vicente González-García, José Antonio Cuchí-Oterino, Belén Lorenzo-Vidal, Jesús Martín-Gil, and Pablo Martín-Ramos. 2022. "Holm Oak (Quercus ilex subsp. ballota (Desf.) Samp.) Bark Aqueous Ammonia Extract for the Control of Invasive Forest Pathogens" International Journal of Molecular Sciences 23, no. 19: 11882. https://doi.org/10.3390/ijms231911882