Disclosing the Biocide Activity of α-Ag2−2xCuxWO4 (0 ≤ x ≤ 0.16) Solid Solutions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Analysis of the Cu Atom in the α-Ag2WO4 Structure
2.1.1. XRD and Rietveld Rietveld Refinement
2.1.2. Theoretical Models
2.1.3. XPS Spectroscopy
2.1.4. ICP-AES Spectroscopy
2.1.5. MR-Raman and ATR-FTIR Spectroscopy
2.1.6. UV–Vis Spectroscopy and PL Emission
2.2. Antibacterial and Antifungal Activities
2.3. DFT Calculations
2.4. Morphology and Surface Composition: What Really Is Important?
2.4.1. Kröger–Vink Notation for the Exposed Surfaces
2.4.2. Electronic and Magnetic Structure of the (010) and (101) Surfaces
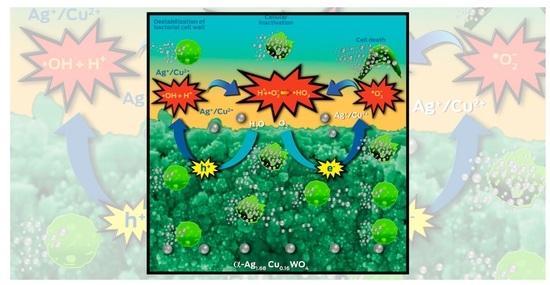
2.5. A Proposed Mechanism for the Biocide Activity
3. Methods and Materials
3.1. Synthesis of α-Ag2−2xCuxWO4 Solid Solutions
3.2. Characterizations of α-Ag2−2xCuxWO4 Solid Solutions
3.3. Antibacterial and Antifungal Activity Measurements
3.4. Theoretical Approach and Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jovanovic, D.J.; Validzic, I.L.; Mitric, M.; Nedeljkovic, J.M. Synthesis and structural characterization of nano-sized copper tungstate particles. Acta Chim. Slov. 2012, 59, 70. [Google Scholar] [PubMed]
- Catlow, C.R.A.; Bromley, S.T.; Hamad, S.; Mora-Fonz, M.; Sokol, A.A.; Woodley, S.M. Modelling nano-clusters and nucleation. Phys. Chem. Chem. Phys. 2010, 12, 786. [Google Scholar] [CrossRef]
- Jacomaci, N.; Junior, E.S.; Oliveira, F.M.B.d.; Longo, E.; Zaghete, M.A. Dielectric behavior of α-Ag2WO4 and its huge dielectric loss tangent. Mater. Res. 2019, 22, 1. [Google Scholar] [CrossRef]
- Tang, J.W.; Ye, J.H. Correlation of crystal structures and electronic structures and photocatalytic properties of the W-containing oxides. J. Mater. Chem. 2005, 15, 4246. [Google Scholar] [CrossRef]
- Sreedevi, A.; Priyanka, K.P.; Babitha, K.K.; Sabu, N.A.; Anu, T.S.; Varghese, T. Chemical synthesis, structural characterization and optical properties of nanophase α-Ag2WO4. Indian J. Phys. 2015, 89, 889. [Google Scholar] [CrossRef]
- Cavalcante, L.S.; Almeida, M.A.; Avansi, W., Jr.; Tranquilin, R.L.; Longo, E.; Batista, N.C.; Mastelaro, V.R.; Li, M.S. Cluster coordination and photoluminescence properties of α-Ag2WO4 microcrystals. Inorg. Chem. 2012, 51, 10675. [Google Scholar] [CrossRef]
- Ramezani, M.; Pourmortazavi, S.M.; Sadeghpur, M.; Yazdani, A.; Kohsari, I. Silver tungstate nanostructures: Electrochemical synthesis and its statistical optimization. J. Mater. Sci. Mater. Electron. 2015, 26, 3861. [Google Scholar] [CrossRef]
- de Santana, Y.V.B.; Gomes, J.E.C.; Matos, L.; Cruvinel, G.H.; Perrin, A.; Perrin, C.; Andres, J.; Varela, J.A.; Longo, E. Silver molybdate and silver tungstate nanocomposites with enhanced photoluminescence. Nanomater. Nanotechnol. 2014, 4, 22. [Google Scholar] [CrossRef]
- Pinatti, I.M.; Nogueira, I.C.; Pereira, W.S.; Pereira, P.F.S.; Gonçalves, R.F.; Varela, J.A.; Longoc, E.; Rosa, I.L.V. Structural and photoluminescence properties of Eu3+ doped α-Ag2WO4 synthesized by the green coprecipitation methodology. Dalton Trans. 2015, 44, 17673. [Google Scholar] [CrossRef]
- Longo, E.; Volanti, D.P.; Longo, V.M.; Gracia, L.; Nogueira, I.C.; Almeida, M.A.P.; Pinheiro, A.N.; Ferrer, M.M.; Cavalcante, L.S.; Andres, J. Toward an understanding of the growth of Ag filaments on α-Ag2WO4 and their photoluminescent properties: A combined experimental and theoretical study. J. Phys. Chem. C 2014, 118, 1229. [Google Scholar] [CrossRef]
- Roca, R.A.; Sczancoski, J.C.; Nogueira, I.C.; Fabbro, M.T.; Alves, H.C.; Gracia, L.; Santos, L.P.S.; de Souza, C.P.; Andres, J.; Luz, G.E., Jr.; et al. Facet-dependent photocatalytic and antibacterial properties of α-Ag2WO4 crystals: Combining experimental data and theoretical insights. Catal. Sci. Techn. 2015, 5, 4091. [Google Scholar] [CrossRef]
- de Foggi, C.C.; de Oliveira, R.C.; Fabbro, M.T.; Vergani, C.E.; Andres, J.; Longo, E.; Machado, A.L. Tuning the morphological, optical, and antimicrobial properties of α-Ag2WO4 microcrystals using different solvents. Cryst. Growth Des. 2017, 17, 6239. [Google Scholar] [CrossRef]
- Nobre, F.X.; Bastos, I.S.; Fontenelle, R.O.S.; Júnior, E.A.A.; Takeno, M.L.; Manzato, L.; Matos, J.M.E.; Orlandi, P.P.; Mendes, J.F.S.; Brito, W.R.; et al. Antimicrobial properties of α-Ag2WO4 rod-like microcrystals synthesized by sonochemistry and sonochemistry followed by hydrothermal conventional method. Ultrasound 2019, 58, 104620. [Google Scholar] [CrossRef] [PubMed]
- Chávez, N.L.H.; Avila, E.D.; Barbuglia, P.A.; de Oliveira, R.C.; de Foggi, C.C.; Longo, E.; Vergani, C.E. Promising effects of silver tungstate microcrystals on fibroblast human cells and three dimensional collagen matrix models a novel non-cytotoxic material to fight oral disease. J. Coll. Surf. B 2018, 170, 505. [Google Scholar] [CrossRef]
- Pellissari, C.V.G.; Vergani, C.E.; Longo, E.; Pavarina, A.C.; Sanitá, P.V.; Siqueira, W.L.; Jorge, J.H. In vitro toxic effect of biomaterials coated with silver tungstate or silver molybdate microcrystals. J. Nanomater. 2020, 2020, 2971827. [Google Scholar] [CrossRef]
- Dutta, D.P.; Singh, A.; Ballal, A.; Tyagi, A.K. High adsorption capacity for cationic dye removal and antibacterial properties of sonochemically synthesized Ag2WO4 nanorods. Eur. J. Inorg. Chem. 2014, 33, 5724. [Google Scholar] [CrossRef]
- Santos, C.J.; Filho, F.M.; Campos, F.L.; Ferreira, C.d.A.; de Barros, A.L.B.; Soares, D.C.F. Ag2WO4 nanoparticles radiolabeled with technetium-99m: A potential new tool for tumor identification and uptake. J. Radioanal. Nucl. Chem. 2020, 323, 51. [Google Scholar] [CrossRef]
- Foggi, C.C.; Fabbro, M.T.; Santos, L.P.S.; de Santana, Y.V.B.; Vergani, C.E.; Machado, A.L.; Cordoncillo, E.; Andres, J.; Longo, E. Synthesis and evaluation of α-Ag2WO4 as novel antifungal agent. Chem. Phys. Lett. 2017, 674, 125. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Wang, J.-D.; Liu, J.-K.; Yang, X.-H.; Lu, Y. Construction of silver tungstate multilevel sphere clusters by controlling the energy distribution on the crystal surface. CrystEngComm 2015, 17, 1129. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Y. Photoactivity and stability of Ag2WO4 for organic degradation in aqueous suspensions. Appl. Surf. Sci. 2014, 319, 319. [Google Scholar] [CrossRef]
- Zhang, R.; Cui, H.; Yang, X.; Liu, H.; Tang, H.; Li, Y. Facile hydrothermal synthesis and photocatalytic activity of rod-like nanosized silver tungstate. Micro Nano Lett. 2012, 7, 1285. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Hashemi, M.M. One pot oxidative cleavage of cyclohexene to adipic acid using silver tungstate nano-rods in a Bronsted acidic ionic liquid. Rsc. Adv. 2015, 5, 31298. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Zhang, G.-Y.; Cui, G.-W.; Xu, Y.-Y.; Liu, Y.; Xing, C.-Y. Controllable fabrication of α-Ag2WO4 nanorod-clusters with superior simulated sunlight photocatalytic performance. Inorg. Chem. Front. 2019, 6, 209. [Google Scholar]
- Neto, N.F.A.; Oliveira, P.M.; Bomio, M.R.D.; Motta, F.V. Effect of temperature on the morphology and optical properties of Ag2WO4 obtained by the co-precipitation method: Photocatalytic activity. Ceram. Int. 2019, 45, 15205. [Google Scholar] [CrossRef]
- Lin, Z.; Li, J.; Zheng, Z.; Yan, J.; Liu, P.; Wang, C.; Yang, G. Electronic Reconstruction of α-Ag2WO4 Nanorods for Visible-Light Photocatalysis. Acs. Nano. 2015, 9, 7256. [Google Scholar] [CrossRef] [PubMed]
- Krtil, P.; Nishimura, S.; Yoshimura, M. Electrochemical formation of ternary oxide films-an EQCM approach to galvanostatic deposition of alkali earth metal tungstates and molybdates. Electrochim. Acta. 1999, 44, 3911. [Google Scholar] [CrossRef]
- Macedo, N.G.; Gouveia, A.F.; Roca, R.A.; Assis, M.; Gracia, L.; Andrés, J.; Leite, E.R.; Longo, E. Surfactant-mediated morphology and photocatalytic activity of α-Ag2WO4 Material. J. Phys. Chem. C 2018, 122, 8667. [Google Scholar] [CrossRef]
- Abinaya, M.; Ramachandran, R.; Chenb, S.-M.; Muthuraj, V. Ultrasonic assisted fabrication of silver tungstate encrusted polypyrrole nanocomposite for effective photocatalytic and electrocatalytic applications. J. Ult. Sonoch. 2020, 64, 104913. [Google Scholar]
- Nubla, K.; Sandhyarani, N. Ag nanoparticles anchored Ag2WO4 nanorods: An efficient methanol tolerant and durable Pt free electro-catalyst toward oxygen reduction reaction. J. Elect. Acta 2020, 340, 135942. [Google Scholar] [CrossRef]
- Song, Q.-W.; Yu, B.; Li, X.-D.; Ma, R.; Diao, Z.-F.; Li, R.-G.; Li, W.; He, L.-N. Efficient chemical fixation of CO2 promoted by a bifunctional Ag2WO4/Ph3P system. Green Chem. 2014, 16, 1633. [Google Scholar] [CrossRef]
- Stone, D.; Liu, J.; Singh, D.P.; Muratore, C.; Voevodin, A.A.; Mishra, S.; Rebholz, C.; Ge, Q.; Aouadi, S.M. Layered atomic structures of double oxides for low shear strength at high temperatures. Scr. Mater. 2010, 62, 735. [Google Scholar] [CrossRef]
- da Silva, L.F.; Catto, A.C.; Avansi, W., Jr.; Cavalcante, L.S.; Andres, J.; Aguir, K.; Mastelaro, V.R.; Longo, E. A novel ozone gas sensor based on one-dimensional (1D) α-Ag2WO4 nanostructures. Nanoscale 2014, 6, 4058. [Google Scholar] [CrossRef] [PubMed]
- Sreedevi, A.; Priyanka, K.P.; Vattappalam, S.C.; Varghese, T. Silver tungstate nanoparticles for the detection of ethanol, ammonia and acetone gases. J. Electron. Mater. 2018, 47, 6328. [Google Scholar] [CrossRef]
- Lusi, M. Engineering crystal properties through solid solutions. Cryst. Growth Des. 2018, 18, 3704. [Google Scholar] [CrossRef]
- Andres, J.; Gracia, L.; Gonzalez-Navarrete, P.; Longo, V.M.; Avansi, W., Jr.; Volanti, D.P.; Ferrer, M.M.; Lemos, P.S.; La Porta, F.A.; Hernandes, A.C.; et al. Structural and electronic analysis of the atomic scale nucleation of Ag on α-Ag2WO4 induced by electron irradiation. Sci. Rep. 2014, 4, 5391. [Google Scholar] [CrossRef]
- Yoong, L.S.; Chong, F.K.; Dutta, B.K. Development of copper-doped TiO2 photocatalyst for hydrogen production under visible light. Energy 2009, 34, 1652. [Google Scholar] [CrossRef]
- Pereira, W.d.S.; Sczancoski, J.C.; Calderon, Y.N.C.; Mastelaro, V.R.; Botelho, G.; Machado, T.R.; Leite, E.R.; Longo, E. Influence of Cu substitution on the structural ordering, photocatalytic activity and photoluminescence emission of Ag3-2xCuxPO4 powders. Appl. Sur. Sci. 2018, 440, 61. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.; Liu, H.; Yang, J.; Liu, Y.; Liu, X.; Gao, M.; Wei, M.; Zhang, X.; Jiang, Y. Structural, optical and magnetic properties of Cu and V co-doped ZnO nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2013, 47, 1–5. [Google Scholar] [CrossRef]
- Ijeh, R.O.; Nwanya, A.C.; Nkele, A.C.; Madiba, I.G.; Bashir, A.K.H.; Ekwealor, A.B.C.; Osuji, R.U.; Maaza, M.; Ezema, F. Optical, electrical and magnetic properties of copper doped electrodeposited MoO3 thin films. Ceram. Int. 2020, 46, 10820. [Google Scholar] [CrossRef]
- Nobre, F.X.; Nogueira, I.C.; Souza, G.d.S.; Matos, J.M.E.d.; Couceiro, P.R.d.C.; Brito, W.R.; de la Cruz, J.P.; Leyet Ruiz, Y. Structural and Optical Properties of Ca0.9Cu0.01WO4 Solid Solution Synthesized by Sonochemistry Method at Room Temperature. Inorg. Chem. 2020, 59, 6039. [Google Scholar] [CrossRef]
- Yourey, J.E.; Kurtz, J.B.; Bartlett, B.M. Structure, optical properties, and magnetism of the full Zn1-xCuxWO4 (0 ≤ x ≤ 1) composition range. Inorg. Chem. 2012, 51, 10394. [Google Scholar] [CrossRef] [PubMed]
- Patureau, P.; Josse, M.; Dessapt, R.; Mevellec, J.Y.; Porcher, F.; Maglione, M.; Deniard, P.; Payen, C. Incorporation of Jahn-Teller Cu2+ Ions into Magnetoelectric Multiferroic MnWO4: Structural, Magnetic, and Dielectric Permittivity Properties of Mn1-xCuxWO4 (x ≤ 0.25). Inorg. Chem. 2015, 54, 10623. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, K.; Nabialek, M.; Szota, M. Influence of Cu Substitution on the Structure and Magnetic Properties of Partially Crystallized Fe62-xCo10Y8MexB20 Alloys. Rev. Chim. 2017, 68, 408. [Google Scholar] [CrossRef]
- Banerjee, D.; Bahadur, D.; Suresh, K.G.; Nigam, A.K. Effect of Cu substitution on the structural and magnetic properties of. Phys. B Condens. Matter 2006, 378, 1091. [Google Scholar] [CrossRef]
- Asokan, K.; Chen, Y.S.; Pao, C.W.; Tsai, H.M.; Lee, C.W.O.; Lin, C.H.; Hsueh, H.C.; Ling, D.C.; Pong, W.F.; Chiou, J.W.; et al. Effect of Co, Ni, and Cu substitution on the electronic structure of hexagonal YMnO3 studied by x-ray absorption spectroscopy. Appl. Phys. Lett. 2009, 95, 131901. [Google Scholar] [CrossRef]
- Din, M.F.M.; Wang, J.L.; Zeng, R.; Shamba, P.; Debnath, J.C.; Dou, S.X. Effects of Cu substitution on structural and magnetic properties of La0.7Pr0.3Fe11.4Si1.6 compounds. Intermetallics 2013, 36, 1–7. [Google Scholar] [CrossRef]
- Mary, T.A.; Kumar, N.R.S.; Varadaraju, U.V. Influence of Cu-site substitution on the structure and superconducting properties of the NdBa2Cu3-xMxO7+δ (M = Fe,Co) and NdBa2Cu3-xMxO7-δ (M = Ni,Zn) system. Phys. Rev. B 1993, 48, 16727. [Google Scholar] [CrossRef]
- Pereira, W.d.S.; Ferrer, M.M.; Botelho, G.; Gracia, L.; Nogueira, I.C.; Pinatti, I.M.; Rosa, I.L.V.; La Porta, F.A.; Andres, J.; Longo, E. Effects of chemical substitution on the structural and optical properties of α-Ag2−2xNixWO4 (0 ≤ x ≤ 0.08) solid solutions. Phys. Chem. Chem. Phys. 2016, 18, 21966. [Google Scholar] [CrossRef]
- Pereira, P.F.S.; Santos, C.C.; Gouveia, A.F.; Ferrer, M.M.; Pinatti, I.M.; Botelho, G.; Sambrano, J.R.; Rosa, I.L.V.; Andres, J.; Longo, E. α-Ag2-2xZnxWO4 (0 ≤ x ≤ 0.25) solid solutions: Structure, morphology, and optical properties. Inorg. Chem. 2017, 56, 7360. [Google Scholar] [CrossRef]
- Silva, M.D.P.; Goncalves, R.F.; Nogueira, I.C.; Longo, V.M.; Mondoni, L.; Moron, M.G.; Santana, Y.V.; Longo, E. Microwave-assisted hydrothermal synthesis of Ag2(W1-xMox)O4 heterostructures: Nucleation of Ag, morphology, and photoluminescence properties. Spectrochim. Acta Part A 2016, 153, 428. [Google Scholar] [CrossRef]
- Penha, M.D.; Gouveia, A.F.; Teixeira, M.M.; de Oliveira, R.C.; Assis, M.; Sambrano, J.R.; Yokaichya, F.; Santos, C.C.; Gonçalves, R.F.; Li, M.S.; et al. Structure, optical properties, and photocatalytic activity of alpha-Ag2W0.75Mo0.25O4. Mater. Res. Bull. 2020, 132, 111011. [Google Scholar] [CrossRef]
- Pinatti, I.M.; Pereira, P.F.S.; de Assis, M.; Longo, E.; Rosa, I.L.V. Rare earth doped silver tungstate for photoluminescent applications. J. Alloys Compd. 2019, 771, 433. [Google Scholar] [CrossRef]
- Nascimento, M.V.B.d.; Nobre, F.X.; de Araújo, E.N.D.; de Araújo, C.I.L.; Couceiro, P.R.C.; Manzato, L. Ag2–xCuxWO4 Solid Solution: Structure, Morphology, Optical Properties, and Photocatalytic Performance in the Degradation of RhB under Blue Light-Emitting Device Irradiation. J. Phys. Chem. C 2021, 125, 11875. [Google Scholar] [CrossRef]
- Ayappan, C.; Palanivel, B.; Jayaraman, V.; Mani, A. Copper ions induced α-Ag2–2xCuxWO4 (0 ≤ x ≤ 0.12) solid solutions with favorable sunlight photocatalytic removal of toxic pollutants. J. Alloy. Compd. 2021, 871, 159530. [Google Scholar] [CrossRef]
- Longo, E.; Cavalcante, L.S.; Volanti, D.P.; Gouveia, A.F.; Longo, V.M.; Varela, J.A.; Orlandi, M.O.; Andres, J. Direct in situ observation of the electron-driven synthesis of Ag filaments on alpha-Ag2WO4 crystals. Sci. Rep. 2013, 3, 1676. [Google Scholar] [CrossRef]
- Larson, A.C.; von Dreele, R.B. General Structure Analysis System (GSAS), Los Alamos National Laboratory Report LAUR 86-748; The Regents of the University of California: California, CA, USA, 2004. [Google Scholar]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano. 2019, 13, 7359. [Google Scholar] [CrossRef]
- Xu, D.; Cheng, B.; Zhang, J.; Wang, W.; Yu, J.; Ho, W. Photocatalytic activity of Ag2MO4 (M = Cr, Mo, W) photocatalysts. J. Mater. Chem. A 2015, 3, 20153. [Google Scholar] [CrossRef]
- Sreedevi, A.; Priyanka, K.P.; Babitha, K.K.; Ganesh, S.; Varghese, T. Influence of electron beam irradiation on structural and optical properties of α-Ag2WO4 nanoparticles. Micron 2016, 88, 1–6. [Google Scholar]
- Turkovi, A.; Fox, D.L.; Scott, J.F.; Geller, S.; Ruse, G.F. High Temperature Raman Spectroscopy of Silver Tetratungstate, Ag8W4016. Mater. Res. Bull. 1977, 12, 189. [Google Scholar] [CrossRef]
- Pereira, P.F.S.; Nogueira, I.C.; Longo, E.; Nassar, E.J.; Rosa, I.L.V.; Cavalcante, L.S. Rietveld refinement and optical properties of SrWO4:Eu3+ powders prepared by the non-hydrolytic sol-gel method. J. Rare Earth. 2015, 33, 113. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. Ein Beitrag Zur Optik Der Farbanstriche. Z. Fur. Tech. Phys. 1931, 12, 593. [Google Scholar]
- Longo, V.M.; Orhan, E.; Cavalcante, L.S.; Porto, S.L.; Espinosa, J.W.M.; Varela, J.A.; Longo, E. Understanding the origin of photoluminescence in disordered Ca0.60Sr0.40WO4: An experimental and first-principles study. Chem. Phys. 2007, 334, 180. [Google Scholar] [CrossRef]
- Kröger, F.A.; Vink, H.J. Relations between the Concentrations of Imperfections in Crystalline Solids. Solid State Phys. 1956, 3, 307. [Google Scholar]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308. [Google Scholar] [CrossRef]
- Baek, Y.W.; An, Y.J. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total Environ. 2011, 409, 1603. [Google Scholar] [CrossRef]
- Santo, C.E.; Taudte, N.; Nies, D.H.; Grass, G. Contribution of copper ion resistance to survival of Escherichia coli on metallic copper surfaces. Appl. Environ. Microbiol. 2008, 74, 977. [Google Scholar] [CrossRef]
- Pramanik, A.; Laha, D.; Bhattacharya, D.; Pramanik, P.; Karmakar, P. A novel study of antibacterial activity of copper iodide nanoparticle mediated by DNA and membrane damage. Colloids Surf. B Biointerfaces 2012, 96, 50. [Google Scholar] [CrossRef]
- Wang, Q.P.; Guo, X.X.; Wu, W.H.; Liu, S.X. Preparation of Fine Ag2WO4 Antibacterial Powders and Its Application in the Sanitary Ceramics. Adv. Mater. Res. 2011, 284–286, 1321. [Google Scholar]
- Mondego, M.; de Oliveira, R.C.; Penha, M.; Li, M.S.; Longo, E. Blue and red light photoluminescence emission at room temperature from CaTiO3 decorated with α-Ag2WO4. Ceram. Int. 2017, 43, 5759. [Google Scholar] [CrossRef]
- Andrés, J.; Gracia, L.; Gouveia, A.F.; Ferrer, M.M.; Longo, E. Effects of surface stability on the morphological transformation of metals and metal oxides as investigated by first-principles calculations. Nanotechnology 2015, 26, 405703. [Google Scholar] [CrossRef]
- Wulff, G., XXV. Zur Frage der Geschwindigkeit des Wachsthums und der Auflösung der Krystallflächen. Z. Kristallogr. 1901, 34, 449. [Google Scholar] [CrossRef]
- Gouveia, A.F.; Gracia, L.; Longo, E.; San-Miguel, M.A.; Andrés, J. Modulating the properties of multifunctional semiconductors by means of morphology: Theory meets experiments. Comput. Mater. Sci. 2021, 188, 110217. [Google Scholar] [CrossRef]
- Ferrer, M.M.; Gouveia, A.F.; Gracia, L.; Longo, E.; Andres, J. A 3D platform for the morphology modulation of materials: First principles calculations on the thermodynamic stability and surface structure of metal oxides: Co3O4, α-Fe2O3, and In2O3. Modell. Simul. Mater. Sci. Eng. 2016, 24, 025007. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Andrés, J.; Gracia, L.; de Oliveira, M.S.M.P.; Mercury, J.M.R.; Longo, E.; Nogueira, I.C. Geometry, electronic structure, morphology, and photoluminescence emissions of BaW1-xMoxO4 (x = 0, 0.25, 0.50, 0.75, and 1) solid solutions: Theory and experiment in concert. Appl. Surf. Sci. 2019, 463, 907. [Google Scholar] [CrossRef]
- Gouveia, A.F.; Ferrer, M.M.; Sambrano, J.R.; Andres, J.; Longo, E. Modeling the atomic-scale structure, stability, and morphological transformations in the tetragonal phase of LaVO4. Chem. Phys. Lett. 2016, 660, 87. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, V.L.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark. Langmuir 2015, 31, 9155. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Hedberg, J.; Blomberg, E.; Odnevall, I. Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media—A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization. Nanomaterials 2022, 12, 1922. [Google Scholar] [CrossRef]
- Wayne, P.; Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; CLSI Document M27-A3; Clinical and Laboratory Standards Institute: Wayne, OA, USA, 2008; Volume 28, p. 6. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically CLSI Document M7-A7; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006; p. 7. [Google Scholar]
- Wady, A.F.; Machado, A.L.; Foggi, C.C.; Zamperini, C.A.; Zucolotto, V.; Moffa, E.B.; Vergani, C.E. Effect of a Silver Nanoparticles Solution on Staphylococcus aureus and Candida spp. J. Nanomater. 2014, 2014, 128. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phy. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initiomolecular dynamics for open-shell transition metals. Phys. Rev. B 1993, 48, 13115. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505. [Google Scholar] [CrossRef]












| α-Ag2−xCuxWO4 | Theoretical (ppm) | Experimental (ppm) |
|---|---|---|
| x = 0.005 | 0.300 | 0.354 |
| x = 0.01 | 0.634 | 0.687 |
| x = 0.02 | 1.118 | 1.379 |
| x = 0.04 | 2.114 | 2.778 |
| x = 0.08 | 4.564 | 5.631 |
| x = 0.16 | 9.860 | 11.574 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, P.F.d.S.; De Foggi, C.C.; Gouveia, A.F.; Pinatti, I.M.; Cabral, L.A.; Guillamon, E.; Sorribes, I.; San-Miguel, M.A.; Vergani, C.E.; Simões, A.Z.; et al. Disclosing the Biocide Activity of α-Ag2−2xCuxWO4 (0 ≤ x ≤ 0.16) Solid Solutions. Int. J. Mol. Sci. 2022, 23, 10589. https://doi.org/10.3390/ijms231810589
Pereira PFdS, De Foggi CC, Gouveia AF, Pinatti IM, Cabral LA, Guillamon E, Sorribes I, San-Miguel MA, Vergani CE, Simões AZ, et al. Disclosing the Biocide Activity of α-Ag2−2xCuxWO4 (0 ≤ x ≤ 0.16) Solid Solutions. International Journal of Molecular Sciences. 2022; 23(18):10589. https://doi.org/10.3390/ijms231810589
Chicago/Turabian StylePereira, Paula Fabiana dos Santos, Camila Cristina De Foggi, Amanda Fernandes Gouveia, Ivo Mateus Pinatti, Luís Antônio Cabral, Eva Guillamon, Iván Sorribes, Miguel A. San-Miguel, Carlos Eduardo Vergani, Alexandre Zirpoli Simões, and et al. 2022. "Disclosing the Biocide Activity of α-Ag2−2xCuxWO4 (0 ≤ x ≤ 0.16) Solid Solutions" International Journal of Molecular Sciences 23, no. 18: 10589. https://doi.org/10.3390/ijms231810589