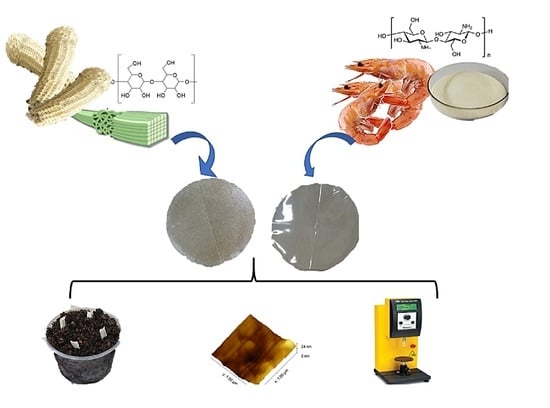
Properties and Biodegradability of Films Based on Cellulose and Cellulose Nanocrystals from Corn Cob in Mixture with Chitosan
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield of Cellulose and Cellulose Nanocrystals
2.2. Films Characterization
2.2.1. Physical Properties
2.2.2. Mechanical Properties
2.2.3. Color Parameters
2.2.4. Films Topography
2.2.5. Films Components Interactions Evaluated by FTIR
2.2.6. Films Biodegradability
2.2.7. Films Compostability
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Cellulose Extraction
3.2.2. Cellulose Nanocrystals Production
3.2.3. Cellulose (C) and Cellulose Nanocrystals (NC) Yield
3.2.4. Nanocellulose Crystals Size
3.2.5. Edible Films Production
3.2.6. Films Characterization
Moisture Content
Water Solubility
Film Thickness
Water Vapor Permeability
Mechanical Properties
Color
Films’ Topography
Fourier Transform Infrared Spectroscopy (FTIR)
Films’ Biodegradability
Films’ Compostability
Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grumezescu, A.M.; Holban, A.M. Food Packaging and Preservation (Handbook of Food Bioengineering); Academic Press: Cambridge, MA, USA, 2018; ISBN 978-0-12-811516-9. [Google Scholar]
- Bridson, J.H.; Gaugler, E.C.; Smith, D.A.; Northcott, G.L.; Gaw, S. Leaching and Extraction of Additives from Plastic Pollution to Inform Environmental Risk: A Multidisciplinary Review of Analytical Approaches. J. Hazard. Mater. 2021, 414, 125571. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.M.M.; Gonçalves, M.P. Strategies to Improve the Mechanical Strength and Water Resistance of Agar Films for Food Packaging Applications. Carbohydr. Polym. 2015, 132, 196–204. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in Bio-Based Food Packaging Applications. Ind. Crops Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Adnan, A.S.; Nurul Fazita, M.R.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.K.M.; Dungani, R. A Review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar] [CrossRef]
- Hammam, A.R.A. Technological, Applications, and Characteristics of Edible Films and Coatings: A Review. SN Appl. Sci. 2019, 1, 632. [Google Scholar] [CrossRef]
- Gupta, P.K.; Raghunath, S.S.; Prasanna, D.V.; Venkat, P.; Shree, V.; Chithananthan, C.; Choudhary, S.; Surender, K.; Geetha, K. An Update on Overview of Cellulose, Its Structure and Applications. In Cellulose; Pascual, A.R., Martín, M.E.E., Eds.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Fang, Z.; Hou, G.; Chen, C.; Hu, L. Nanocellulose-Based Films and Their Emerging Applications. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100764. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S. Cellulose Nanocrystals: Synthesis, Functional Properties, and Applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose Properties and Applications in Colloids and Interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global Maize Production, Consumption and Trade: Trends and R&D Implications. Food Secur. 2022, 1–25. [Google Scholar] [CrossRef]
- Guo, X.; Lü, X. Chapter 2—The Need for Biofuels in the Context of Energy Consumption. In Advances in 2nd Generation of Bioethanol Production; Lü, X., Ed.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2021; pp. 9–30. ISBN 978-0-12-818862-0. [Google Scholar]
- Kumar, S.; Negi, Y.S.; Upadhyaya, J.S. Studies on Characterization of Corn Cob Based Nanoparticles. Adv. Mater. Lett. 2010, 1, 246–253. [Google Scholar]
- Córdoba, J.A.; Salcedo, E.; Rodríguez, R.; Zamora, J.F.; Manríquez, R.; Contreras, H.; Robledo, J.; Delgado, E. Caracterización y valoración química del olote: Degradación hidrotérmica bajo condiciones subcríticas. Rev. Latinoam. Quím. 2013, 41, 171–184. [Google Scholar]
- Pointner, M.; Kuttner, P.; Obrlik, T.; Jäger, A.; Kahr, H. Composition of Corncobs as a Substrate for Fermentation of Biofuels. Agron. Res. 2014, 12, 391–396. [Google Scholar]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent Progress in Cellulose Nanocrystals: Sources and Production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Velázquez, G.; Vázquez, M. Regenerated Cellulose Films Combined with Glycerol and Polyvinyl Alcohol: Effect of Moisture Content on the Physical Properties. Food Hydrocoll. 2020, 103, 105657. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Lopez-Sanchez, P.; Yang, X. Characterizations of Bacterial Cellulose Nanofibers Reinforced Edible Films Based on Konjac Glucomannan. Int. J. Biol. Macromol. 2020, 145, 634–645. [Google Scholar] [CrossRef]
- Nur Hazirah, M.A.S.P.; Isa, M.I.N.; Sarbon, N.M. Effect of Xanthan Gum on the Physical and Mechanical Properties of Gelatin-Carboxymethyl Cellulose Film Blends. Food Packag. Shelf Life 2016, 9, 55–63. [Google Scholar] [CrossRef]
- Deng, L.L.; Alexander, A.A.; Lei, S.; Anderson, J.S. The Cell Wall Teichuronic Acid Synthetase (TUAS) Is an Enzyme Complex Located in the Cytoplasmic Membrane of Micrococcus Luteus. Biochem. Res. Int. 2010, 2010, 395758. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Wang, Q.; Xu, J.; Fan, X.; Wang, P.; Yuan, J. Biological–Chemical Modification of Cellulose Nanocrystal to Prepare Highly Compatible Chitosan-Based Nanocomposites. Cellulose 2019, 26, 5267–5279. [Google Scholar] [CrossRef]
- Sabbah, M.; Di Pierro, P.; Cammarota, M.; Dell’Olmo, E.; Arciello, A.; Porta, R. Development and Properties of New Chitosan-Based Films Plasticized with Spermidine and/or Glycerol. Food Hydrocoll. 2019, 87, 245–252. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-Based Films and Coatings for Food Packaging: A Review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Development and Evaluation of Chitosan Based Active Nanocomposite Films Containing Bacterial Cellulose Nanocrystals and Silver Nanoparticles. Food Hydrocoll. 2018, 84, 414–423. [Google Scholar] [CrossRef]
- Kassab, Z.; Aziz, F.; Hannache, H.; Ben Youcef, H.; El Achaby, M. Improved Mechanical Properties of K-Carrageenan-Based Nanocomposite Films Reinforced with Cellulose Nanocrystals. Int. J. Biol. Macromol. 2019, 123, 1248–1256. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Dufresne, A.; Pinheiro, I.F.; Souza, D.H.S.; Gouveia, R.F.; Mei, L.H.I.; Lona, L.M.F. How Do Cellulose Nanocrystals Affect the Overall Properties of Biodegradable Polymer Nanocomposites: A Comprehensive Review. Eur. Polym. J. 2018, 108, 274–285. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Tapia, M.S.; Pérez, E.; Famá, L. Structural and Mechanical Properties of Edible Films Made from Native and Modified Cush-Cush Yam and Cassava Starch. Food Hydrocoll. 2015, 45, 211–217. [Google Scholar] [CrossRef]
- Ng, H.-M.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Hui, D.; Low, C.-Y.; Rahmat, A.R. Extraction of Cellulose Nanocrystals from Plant Sources for Application as Reinforcing Agent in Polymers. Compos. Part B Eng. 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Boukouvala, C.; Daniel, J.; Ringe, E. Approaches to Modelling the Shape of Nanocrystals. Nano Converg. 2021, 8, 26. [Google Scholar] [CrossRef]
- Börjesson, M.; Westman, G. Crystalline Nanocellulose—Preparation, Modification, and Properties; IntechOpen: London, UK, 2015; ISBN 978-953-51-2229-6. [Google Scholar]
- Bonardd, S.; Robles, E.; Barandiaran, I.; Saldías, C.; Leiva, Á.; Kortaberria, G. Biocomposites with Increased Dielectric Constant Based on Chitosan and Nitrile-Modified Cellulose Nanocrystals. Carbohydr. Polym. 2018, 199, 20–30. [Google Scholar] [CrossRef]
- Valizadeh, S.; Naseri, M.; Babaei, S.; Hosseini, S.M.H.; Imani, A. Development of Bioactive Composite Films from Chitosan and Carboxymethyl Cellulose Using Glutaraldehyde, Cinnamon Essential Oil and Oleic Acid. Int. J. Biol. Macromol. 2019, 134, 604–612. [Google Scholar] [CrossRef]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable Polymers and Green-Based Antimicrobial Packaging Materials: A Mini-Review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent Developments in Antibacterial and Antifungal Chitosan and Its Derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Bats, I.; Di Pierro, P.; Villalonga-Santana, R.; Garcia-Almendarez, B.; Porta, R. Bioactive Mesoporous Silica Nanocomposite Films Obtained from Native and Transglutaminase-Crosslinked Bitter Vetch Proteins. Food Hydrocoll. 2018, 82, 106–115. [Google Scholar] [CrossRef]
- Gutiérrez, T.J. Are Modified Pumpkin Flour/Plum Flour Nanocomposite Films Biodegradable and Compostable? Food Hydrocoll. 2018, 83, 397–410. [Google Scholar] [CrossRef]
- Kalita, N.K.; Bhasney, S.M.; Mudenur, C.; Kalamdhad, A.; Katiyar, V. End-of-Life Evaluation and Biodegradation of Poly(Lactic Acid) (PLA)/Polycaprolactone (PCL)/Microcrystalline Cellulose (MCC) Polyblends under Composting Conditions. Chemosphere 2020, 247, 125875. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, Ó.J.; Ospina, D.A.; Montoya, S. Compost Supplementation with Nutrients and Microorganisms in Composting Process. Waste Manag. 2017, 69, 136–153. [Google Scholar] [CrossRef]
- Dean, K.; Sangwan, P.; Way, C.; Zhang, X.; Martino, V.P.; Xie, F.; Halley, P.J.; Pollet, E.; Avérous, L. Glycerol Plasticised Chitosan: A Study of Biodegradation via Carbon Dioxide Evolution and Nuclear Magnetic Resonance. Polym. Degrad. Stab. 2013, 98, 1236–1246. [Google Scholar] [CrossRef]
- Salehpour, S.; Jonoobi, M.; Ahmadzadeh, M.; Siracusa, V.; Rafieian, F.; Oksman, K. Biodegradation and Ecotoxicological Impact of Cellulose Nanocomposites in Municipal Solid Waste Composting. Int. J. Biol. Macromol. 2018, 111, 264–270. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, P. Improvement of Mechanical Properties and Thermal Stability of Biodegradable Rice Starch–Based Films Blended with Carboxymethyl Chitosan. Ind. Crops Prod. 2018, 122, 37–48. [Google Scholar] [CrossRef]
- Melikoğlu, A.Y.; Bilek, S.E.; Cesur, S. Optimum Alkaline Treatment Parameters for the Extraction of Cellulose and Production of Cellulose Nanocrystals from Apple Pomace. Carbohydr. Polym. 2019, 215, 330–337. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, S.; Ma, L.; Yu, Y.; Dai, H.; Zhang, Y. Extraction and Comparison of Cellulose Nanocrystals from Lemon (Citrus Limon) Seeds Using Sulfuric Acid Hydrolysis and Oxidation Methods. Carbohydr. Polym. 2020, 238, 116180. [Google Scholar] [CrossRef]
- Gupta, M.; Ho, D.; Santoro, D.; Torfs, E.; Doucet, J.; Vanrolleghem, P.A.; Nakhla, G. Experimental Assessment and Validation of Quantification Methods for Cellulose Content in Municipal Wastewater and Sludge. Environ. Sci. Pollut. Res. 2018, 25, 16743–16753. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; Zhu, S.; Wu, Q.; Li, J.; Gao, Y.; Wang, B.; Li, J.; Gao, W.; Zeng, J.; et al. Preparation of Nanocellulose in High Yield via Chemi-Mechanical Synergy. Carbohydr. Polym. 2021, 251, 117094. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-García, M.; Calderón-Domínguez, G.; Chanona-Pérez, J.J.; Mendoza-Madrigal, A.G.; Di Pierro, P.; García-Almendárez, B.E.; Amaro-Reyes, A.; Regalado-González, C. Physical, Structural, Barrier, and Antifungal Characterization of Chitosan-Zein Edible Films with Added Essential Oils. Int. J. Mol. Sci. 2017, 18, 2370. [Google Scholar] [CrossRef] [Green Version]
- Escamilla-García, M.; Ríos-Romo, R.A.; Melgarejo-Mancilla, A.; Díaz-Ramírez, M.; Hernández-Hernández, H.M.; Amaro-Reyes, A.; Pierro, P.D.; Regalado-González, C. Rheological and Antimicrobial Properties of Chitosan and Quinoa Protein Filmogenic Suspensions with Thyme and Rosemary Essential Oils. Foods 2020, 9, 1616. [Google Scholar] [CrossRef]
- Ghosh, T.; Nakano, K.; Katiyar, V. Curcumin Doped Functionalized Cellulose Nanofibers Based Edible Chitosan Coating on Kiwifruits. Int. J. Biol. Macromol. 2021, 184, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Huang, J.; Zheng, X.; Liu, S.; Lu, K.; Tang, K.; Liu, J. Heat Sealable Soluble Soybean Polysaccharide/Gelatin Blend Edible Films for Food Packaging Applications. Food Packag. Shelf Life 2020, 24, 100485. [Google Scholar] [CrossRef]
- Escamilla-García, M.; Delgado-Sánchez, L.F.; Ríos-Romo, R.A.; García-Almendárez, B.E.; Calderón-Domínguez, G.; Méndez-Méndez, J.V.; Amaro-Reyes, A.; Di Pierro, P.; Regalado-González, C. Effect of Transglutaminase Cross-Linking in Protein Isolates from a Mixture of Two Quinoa Varieties with Chitosan on the Physicochemical Properties of Edible Films. Coatings 2019, 9, 736. [Google Scholar] [CrossRef]
- ASTM G21-09; Standard Practice for Determining Resistance of Synthetic Polymeric Materials to Fungi. ASTM International: West Conshohocken, PA, USA, 2013. Available online: https://www.astm.org/g0021-09.html (accessed on 17 July 2022).
- Sintim, H.Y.; Bary, A.I.; Hayes, D.G.; English, M.E.; Schaeffer, S.M.; Miles, C.A.; Zelenyuk, A.; Suski, K.; Flury, M. Release of Micro- and Nanoparticles from Biodegradable Plastic during in Situ Composting. Sci. Total Environ. 2019, 675, 686–693. [Google Scholar] [CrossRef]






| Film | MC (% w/w) | Ws (% w/w) | WVP × 1012 (g m−1 s−1 Pa−1) |
|---|---|---|---|
| C-CH | 20.07 ± 1.02 a | 17.44 ± 0.57 a | 1.57 ± 0.10 a |
| C-NC-CH | 17.19 ± 1.11 b | 19.80 ± 1.62 a | 1.05 ± 0.15 b |
| CH | 33.35 ± 1.20 c | 35.73 ± 6.27 b | 7.8 ± 0.2 c |
| Films | Thickness (μm) | Tensile Strength (MPa) |
|---|---|---|
| C-CH | 615.93 ± 30.03 a | 0.793 ± 0.228 a |
| C-NC-CH | 632.70 ± 15.4 a | 0.836 ± 0.129 a |
| CH | 474.90 ± 46.27 b | 1.093 ± 0.250 a |
| Film | a* | b* | L* | ΔE |
|---|---|---|---|---|
| C-CH | −1.88 ± 0.02 a | 19.09 ± 0.01 a | 87.67 ± 0.17 a | 19.42 ± 0.04 a |
| C-NC-CH | −0.95 ± 0.02 b | 19.18 ± 0.02 b | 89.80 ± 0.66 b | 19.21 ± 0.04 b |
| CH | −0.96 ± 0.00 b | 7.42 ± 0.01 c | 97.69 ± 0.04 c | 10.08 ± 0.03 c |
| Films | Compostability (%) |
|---|---|
| C-CH | 37.35 ± 1.88 a |
| C-NC-CH | 43.75 ± 7.18 a |
| CH | 40.74 ± 19.36 a |
| FP | 100 ± 0.10 b |
| Growth Observation * | Scale |
|---|---|
| None | 0 |
| Trace growth (<10%) | 1 |
| Light growth (10–30%) | 2 |
| Medium growth (30–60%) | 3 |
| High growth (60%-fully covered) | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escamilla-García, M.; García-García, M.C.; Gracida, J.; Hernández-Hernández, H.M.; Granados-Arvizu, J.Á.; Di Pierro, P.; Regalado-González, C. Properties and Biodegradability of Films Based on Cellulose and Cellulose Nanocrystals from Corn Cob in Mixture with Chitosan. Int. J. Mol. Sci. 2022, 23, 10560. https://doi.org/10.3390/ijms231810560
Escamilla-García M, García-García MC, Gracida J, Hernández-Hernández HM, Granados-Arvizu JÁ, Di Pierro P, Regalado-González C. Properties and Biodegradability of Films Based on Cellulose and Cellulose Nanocrystals from Corn Cob in Mixture with Chitosan. International Journal of Molecular Sciences. 2022; 23(18):10560. https://doi.org/10.3390/ijms231810560
Chicago/Turabian StyleEscamilla-García, Monserrat, Mónica Citlali García-García, Jorge Gracida, Hilda María Hernández-Hernández, José Ángel Granados-Arvizu, Próspero Di Pierro, and Carlos Regalado-González. 2022. "Properties and Biodegradability of Films Based on Cellulose and Cellulose Nanocrystals from Corn Cob in Mixture with Chitosan" International Journal of Molecular Sciences 23, no. 18: 10560. https://doi.org/10.3390/ijms231810560