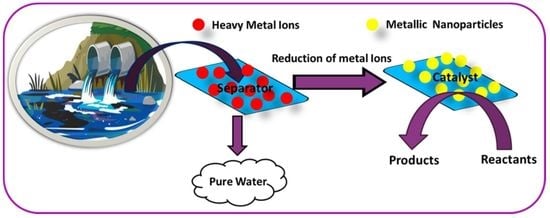
Review on the Use of Heavy Metal Deposits from Water Treatment Waste towards Catalytic Chemical Syntheses
Abstract
:1. Introduction
- Toxic heavy metals (e.g., −Hg, Cr. Pb, As, Sr, Si, Ag, and Ti);
- Radioactive metals (e.g., Tc, U, Rn, Th, Ra, and Am);
- Metals essential for metabolism (e.g., Mo, K, Ca, Fe, Ni, Cu, and Zn);
- Metals detection of biologic effectiveness (e.g., Ge, Sb, Te, Po, and B).
2. Source of Heavy Metals
2.1. Natural Resources
2.2. Mining Operations
2.3. Electronic Waste
2.4. Power Plants
2.5. Agriculture Sources
2.6. Industrial Sources
2.7. Domestic Waste
2.8. Other Sources
3. Concentration of Heavy Metals
3.1. Heavy Metal Concentration in Wastewater
3.2. Heavy Metal Concentration in Sludge
3.3. Regulatory Limits of Heavy Metals
4. Harmful Effect of Heavy Metals on Human Health
5. Recovery of Heavy Metals from Wastewater
5.1. Electrochemical Treatment
5.1.1. Electrocoagulation
5.1.2. Electrodeposition
5.1.3. Electroflotation
5.2. Physico-Chemical Process
5.2.1. Chemical Precipitation
5.2.2. Ion Exchange
5.3. Absorption Process
5.3.1. Activated Carbon (AC)
5.3.2. Carbon Nanotubes (CNTs)
5.3.3. Wood Sawdust
5.4. Current Methods
5.4.1. Membrane Filtration
5.4.2. Photocatalytic Process
5.4.3. Nanotechnology
6. Effect on the Characteristic Water Properties in Presence of Heavy Metals and Their Removal
6.1. Effect of pH
6.2. Effect of Temperature
6.3. Effect of Ionic Strength
6.4. Effect of Natural Organic Matter
7. Recent Progress in Reutilization of Recovered Heavy Metals from Wastewater in Catalytic Chemical Syntheses
8. Research Challenges
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2CP | 2-chloro phenol |
| 3D | three dimensional |
| 4AP | 4-aminophenol |
| 4NP | 4-nitrophenol |
| AC | activated carbon |
| ALG | sodium alginate |
| CNTs | carbon nanotubes |
| CB | conduction band |
| CR | Congo red |
| ED | electrodialysis |
| EPA | Environmental Protection Agency |
| EU | European Union |
| FAO | Food and Agriculture Organization |
| Na-SGS | geopolymer microsphere |
| LDO | Mg/Al-CO3 hydrotalcite |
| MO | methyl orange |
| MB | methylene blue |
| MgFe2O4 | magnesium ferrite |
| MF | microfiltration |
| NaBH4 | sodium borohydride |
| NF | nanofiltration |
| NPs | nanoparticles |
| OSHA | Occupational Safety and Health Administration |
| PAA | poly(amic acid) |
| (P(AAm) | poly(acrylamide) |
| P(MAA) | poly(methacrylic acid) |
| PAHs | polycyclic aromatic hydrocarbons |
| PEI | polyethyleneimine |
| P(APTMACl) | poly(3-acrylamidopropyltrimethyl ammonium chloride |
| PPy-MAA | polypyrrole-mercaptoacetic acid |
| RO | reverse osmosis |
| SF | silk fibroin |
| UF | ultrafiltration |
| VB | valence band |
| WHO | World Health Organization |
References
- Long, Z.; Huang, Y.; Zhang, W.; Shi, Z.; Yu, D.; Chen, Y.; Liu, C.; Wang, R. Effect of different industrial activities on soil heavy metal pollution, ecological risk, and health risk. Environ. Monitor. Assess. 2021, 193, 1–12. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; Von Gunten, U.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef]
- Deng, F.; Luo, X.-B.; Ding, L.; Luo, S.-L. Application of nanomaterials and nanotechnology in the reutilization of metal ion from wastewater. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Luo, X., Deng, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–178. [Google Scholar]
- Akpor, O.B.; Ohiobor, G.O.; Olaolu, D. Heavy metal pollutants in wastewater effluents: Sources, effects and remediation. Adv. Biosci. Bioeng. 2014, 2, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, N.; Majumder, C. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard. Mater. 2008, 151, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Shahzad, S.; Munir, A.; Nadagouda, M.N.; Khan, G.S.; Shams, D.F.; Dionysiou, D.D.; Rana, U.A. Micelles as soil and water decontamination agents. Chem. Rev. 2016, 116, 6042–6074. [Google Scholar] [CrossRef]
- Sheldon, R.A. Fundamentals of green chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451. [Google Scholar] [CrossRef] [Green Version]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM 21 selection guide of classical-and less classical-solvents. Green Chem. 2015, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Varma, R.S. Greener and sustainable trends in synthesis of organics and nanomaterials. ACS Sustain. Chem. Eng. 2016, 4, 5866–5878. [Google Scholar] [CrossRef]
- Tamjidi, S.; Esmaeili, H.; Moghadas, B.K. Application of magnetic adsorbents for removal of heavy metals from wastewater: A review study. Mater. Res. Express 2019, 6, 102004. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M.; Citak, D.; Soylak, M. Equilibrium, kinetic and thermodynamic studies of adsorption of Pb(II) from aqueous solution onto Turkish kaolinite clay. J. Hazard. Mater. 2007, 149, 283–291. [Google Scholar] [CrossRef]
- Ramos, R.L.; Jacome, L.B.; Barron, J.M.; Rubio, L.F.; Coronado, R.G. Adsorption of zinc(II) from an aqueous solution onto activated carbon. J. Hazard. Mater. 2002, 90, 27–38. [Google Scholar] [CrossRef]
- Abd El Hameed, A.H.; Eweda, W.E.; Abou-Taleb, K.A.; Mira, H. Biosorption of uranium and heavy metals using some local fungi isolated from phosphatic fertilizers. Ann. Agric. Sci. 2015, 60, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Huang, S.; Liao, Q.; Hua, M.; Wu, X.; Bi, K.; Yan, C.; Chen, B.; Zhang, X. Survey of heavy metal pollution and assessment of agricultural soil in Yangzhong district, Jiangsu Province, China. Chemosphere 2007, 67, 2148–2155. [Google Scholar] [CrossRef]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Harmens, H.; Buse, A.; Büker, P.; Norris, D.; Mills, G.; Williams, B.; Reynolds, B.; Ashenden, T.W.; Rühling, Å.; Steinnes, E. Heavy metal concentrations in European mosses: 2000/2001 survey. J. Atmos. Chem. 2004, 49, 425–436. [Google Scholar] [CrossRef]
- Radwan, M.A.; Salama, A.K. Market basket survey for some heavy metals in Egyptian fruits and vegetables. Food Chem. Toxicol. 2006, 44, 1273–1278. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [Green Version]
- Belov, D.S.; Mathivathanan, L.; Beazley, M.J.; Martin, W.B.; Bukhryakov, K.V. Stereospecific Ring-Opening Metathesis Polymerization of Norbornene Catalyzed by Iron Complexes. Angew. Chem. Int. Ed. 2021, 60, 2934–2938. [Google Scholar] [CrossRef]
- Poater, A.; Pump, E.; Vummaleti, S.V.C.; Cavallo, L. The activation mechanism of Fe-based olefin metathesis catalysts. Chem. Phys. Lett. 2014, 610–611, 29–32. [Google Scholar] [CrossRef]
- Poater, A.; Vummaleti, S.V.C.; Pump, E.; Cavallo, L. Comparing Ru and Fe-catalyzed olefin metathesis. Dalton Trans. 2014, 43, 11216–11220. [Google Scholar] [CrossRef] [PubMed]
- Luque-Urrutia, J.A.; Gimferrer, M.; Casals-Cruañas, E.; Poater, A. In Silico Switch from Second-to First-Row Transition Metals in Olefin Metathesis: From Ru to Fe and from Rh to Co. Catalysts 2017, 7, 389. [Google Scholar] [CrossRef] [Green Version]
- González-Belman, O.F.; Jiménez-Halla, J.O.C.; Nahra, F.; Cazin, C.S.J.; Poater, A. The role of the metal in the dual-metal catalysed hydrophenoxylation of diphenylacetylene. Catal. Sci. Technol. 2018, 8, 3638–3648. [Google Scholar] [CrossRef]
- Lator, A.; Gaillard, S.; Poater, A.; Renaud, J.-L. Iron-Catalyzed Chemoselective Reduction of α, β-Unsaturated Ketones. Chem. Eur. J. 2018, 24, 5770–5774. [Google Scholar] [CrossRef]
- Gimferrer, M.; Salvador, P.; Poater, A. Computational monitoring of oxidation states in olefin metathesis. Organometallics 2019, 38, 4585–4592. [Google Scholar] [CrossRef]
- Azofra, L.M.; Poater, A. Diastereoselective diazenyl formation: The key for manganese-catalysed alcohol conversion into (E)-alkenes. Dalton Trans. 2019, 48, 14122–14127. [Google Scholar] [CrossRef]
- Luque-Urrutia, J.A.; Pèlachs, T.; Solà, M.; Poater, A. Double-Carrousel Mechanism for Mn-Catalyzed Dehydrogenative Amide Synthesis from Alcohols and Amines. ACS Catal. 2021, 11, 6155–6161. [Google Scholar] [CrossRef]
- Kaur, S.; Kumar, V.; Chawla, M.; Cavallo, L.; Poater, A.; Upadhyay, N. Pesticides curbing soil fertility: Effect of complexation of free metal ions. Front. Chem. 2017, 5, 43. [Google Scholar] [CrossRef] [Green Version]
- Mulchandani, A.; Westerhoff, P. Recovery opportunities for metals and energy from sewage sludges. Bioresour. Technol. 2016, 215, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Ovais, M.; Raza, A.; Naz, S.; Islam, N.U.; Khalil, A.T.; Ali, S.; Khan, M.A.; Shinwari, Z.K. Current state and prospects of the phytosynthesized colloidal gold nanoparticles and their applications in cancer theranostics. Appl. Microbiol. Biotechnol. 2017, 101, 3551–3565. [Google Scholar] [CrossRef]
- Das, T.K.; Remanan, S.; Ghosh, S.; Ghosh, S.K.; Das, N.C. Efficient synthesis of catalytic active silver nanoparticles illuminated cerium oxide nanotube: A mussel inspired approach. Environ. Nanotechnol. Monitor. Manag. 2021, 15, 100411. [Google Scholar] [CrossRef]
- Das, T.K.; Ganguly, S.; Bhawal, P.; Remanan, S.; Mondal, S.; Das, N. Mussel inspired green synthesis of silver nanoparticles-decorated halloysite nanotube using dopamine: Characterization and evaluation of its catalytic activity. Appl. Nanosci. 2018, 8, 173–186. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Z.; Xu, X.; Wang, Y.; Wang, Y.; Huang, F.; Lin, Z. Treatment of nanowaste via fast crystal growth: With recycling of nano-SnO2 from electroplating sludge as a study case. J. Hazard. Mater. 2012, 211, 414–419. [Google Scholar] [CrossRef]
- Das, R.; Giri, S.; Muliwa, A.M.; Maity, A. High-performance Hg(II) removal using thiol-functionalized polypyrrole (PPy/MAA) composite and effective catalytic activity of Hg(II)-adsorbed waste material. ACS Sustain. Chem. Eng. 2017, 5, 7524–7536. [Google Scholar] [CrossRef]
- Das, T.K.; Ganguly, S.; Ghosh, S.; Remanan, S.; Ghosh, S.K.; Das, N.C. In-situ synthesis of magnetic nanoparticle immobilized heterogeneous catalyst through mussel mimetic approach for the efficient removal of water pollutants. Colloid Interface Sci. Commun. 2019, 33, 100218. [Google Scholar] [CrossRef]
- Das, T.K.; Bhawal, P.; Ganguly, S.; Mondal, S.; Das, N.C. A facile green synthesis of amino acid boosted Ag decorated reduced graphene oxide nanocomposites and its catalytic activity towards 4-nitrophenol reduction. Surf. Interface 2018, 13, 79–91. [Google Scholar] [CrossRef]
- Mikula, K.; Izydorczyk, G.; Skrzypczak, D.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Value-added strategies for the sustainable handling, disposal, or value-added use of copper smelter and refinery wastes. J. Hazard. Mater. 2021, 403, 123602. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Xu, C.; An, Z.; Wang, S. Analysis and evaluation of the source of heavy metals in water of the River Changjiang. Environ. Monitor. Assess. 2011, 173, 301–313. [Google Scholar] [CrossRef]
- Bradl, H. Heavy Metals in the Environment: Origin, Interaction and Remediation; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Cheng, H.; Hu, Y.; Luo, J.; Xu, B.; Zhao, J. Geochemical processes controlling fate and transport of arsenic in acid mine drainage (AMD) and natural systems. J. Hazard. Mater. 2009, 165, 13–26. [Google Scholar] [CrossRef]
- Fei, J.-C.; Min, X.-B.; Wang, Z.-X.; Pang, Z.-h.; Liang, Y.-J.; Ke, Y. Health and ecological risk assessment of heavy metals pollution in an antimony mining region: A case study from South China. Environ. Sci. Pollut. Res. 2017, 24, 27573–27586. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xie, X.; Wang, P.; Hu, Y.; Cheng, H. Heavy metal pollution caused by small-scale metal ore mining activities: A case study from a polymetallic mine in South China. Sci. Total Environ. 2018, 639, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Diami, S.M.; Kusin, F.M.; Madzin, Z. Potential ecological and human health risks of heavy metals in surface soils associated with iron ore mining in Pahang, Malaysia. Environ. Sci. Pollut. Res. 2016, 23, 21086–21097. [Google Scholar] [CrossRef]
- Nekouei, R.K.; Pahlevani, F.; Assefi, M.; Maroufi, S.; Sahajwalla, V. Selective isolation of heavy metals from spent electronic waste solution by macroporous ion-exchange resins. J. Hazard. Mater. 2019, 371, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M. Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manag. 2016, 57, 64–90. [Google Scholar] [CrossRef]
- Fujimori, T.; Takigami, H. Pollution distribution of heavy metals in surface soil at an informal electronic-waste recycling site. Environ. Geochem. Health 2014, 36, 159–168. [Google Scholar] [CrossRef]
- Li, J.; Duan, H.; Shi, P. Heavy metal contamination of surface soil in electronic waste dismantling area: Site investigation and source-apportionment analysis. Waste Manag. Res. 2011, 29, 727–738. [Google Scholar]
- Nithya, R.; Sivasankari, C.; Thirunavukkarasu, A. Electronic waste generation, regulation and metal recovery: A review. Environ. Chem. Lett. 2021, 19, 1347–1368. [Google Scholar] [CrossRef]
- Santonicola, S.; De Felice, A.; Cobellis, L.; Passariello, N.; Peluso, A.; Murru, N.; Ferrante, M.C.; Mercogliano, R. Comparative study on the occurrence of polycyclic aromatic hydrocarbons in breast milk and infant formula and risk assessment. Chemosphere 2017, 175, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huo, X.; Liu, Y.; Zhang, Y.; Qin, Q.; Xu, X. Hearing loss risk and DNA methylation signatures in preschool children following lead and cadmium exposure from an electronic waste recycling area. Chemosphere 2020, 246, 125829. [Google Scholar] [CrossRef]
- Seith, R.; Arain, A.L.; Nambunmee, K.; Adar, S.D.; Neitzel, R.L. Self-reported health and metal body burden in an electronic waste recycling community in Northeastern Thailand. J. Occup. Environ. Med. 2019, 61, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Liu, G.; Mian, M.M.; Sun, M.; Wu, D. Characteristics and speciation of heavy metals in fly ash and FGD gypsum from Chinese coal-fired power plants. Fuel 2019, 251, 593–602. [Google Scholar] [CrossRef]
- Zhu, C.; Tian, H.; Cheng, K.; Liu, K.; Wang, K.; Hua, S.; Gao, J.; Zhou, J. Potentials of whole process control of heavy metals emissions from coal-fired power plants in China. J. Clean. Prod. 2016, 114, 343–351. [Google Scholar] [CrossRef]
- Sankhla, M.S.; Kumari, M.; Nandan, M.; Kumar, R.; Agrawal, P. Heavy metals contamination in water and their hazardous effect on human health-a review. Int. J. Curr. Microbiol. App. Sci. 2016, 5, 759–766. [Google Scholar] [CrossRef]
- Sushil, S.; Batra, V.S. Analysis of fly ash heavy metal content and disposal in three thermal power plants in India. Fuel 2006, 85, 2676–2679. [Google Scholar] [CrossRef]
- Singh, R.; Ahirwar, N.K.; Tiwari, J.; Pathak, J. Review on sources and effect of heavy metal in soil: Its bioremediation. Int. J. Res. Appl. Nat. Soc. Sci. 2018, 2008, 1–22. [Google Scholar]
- Guan, Q.; Wang, F.; Xu, C.; Pan, N.; Lin, J.; Zhao, R.; Yang, Y.; Luo, H. Source apportionment of heavy metals in agricultural soil based on PMF: A case study in Hexi Corridor, northwest China. Chemosphere 2018, 193, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Xing, M.; Yang, J. Speciation and transformation of heavy metals during vermicomposting of animal manure. Bioresour. Technol. 2016, 209, 397–401. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, V.K.; Kumar, A.; Sharma, B. Synergistic effects of heavy metals and pesticides in living systems. Front. Chem. 2017, 5, 70. [Google Scholar] [CrossRef]
- Agarwal, S.K. Heavy Metal Pollution; APH Publishing Corporation: New Delhi, India, 2009; Volume 4. [Google Scholar]
- Hejabi, A.T.; Basavarajappa, H.; Karbassi, A.; Monavari, S. Heavy metal pollution in water and sediments in the Kabini River, Karnataka, India. Environ. Monitor. Assess. 2011, 182, 1–13. [Google Scholar] [CrossRef]
- Harada, H.; Kurauchi, M.; Hayashi, R.; Eki, T. Shortened lifespan of nematode Caenorhabditis elegans after prolonged exposure to heavy metals and detergents. Ecotoxicol. Environ. Saf. 2007, 66, 378–383. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Chen, Y.; Korshin, G.V. Effects of varying temperatures and alkalinities on the corrosion and heavy metal release from low-lead galvanized steel. Environ. Sci. Pollut. Res. 2020, 27, 2412–2422. [Google Scholar] [CrossRef]
- Hwang, H.-M.; Fiala, M.J.; Park, D.; Wade, T.L. Review of pollutants in urban road dust and stormwater runoff: Part 1. Heavy metals released from vehicles. Int. J. Urban Sci. 2016, 20, 334–360. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, K.; Wu, W.; Tian, H.; Yi, P.; Zhi, G.; Fan, J.; Liu, S. Atmospheric emissions of typical toxic heavy metals from open burning of municipal solid waste in China. Atmos. Environ. 2017, 152, 6–15. [Google Scholar] [CrossRef]
- Chipasa, K.B. Accumulation and fate of selected heavy metals in a biological wastewater treatment system. Waste Manag. 2003, 23, 135–143. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Vo, D.-V.N. Critical review on hazardous pollutants in water environment: Occurrence, monitoring, fate, removal technologies and risk assessment. Sci. Total Environ. 2021, 797, 149134. [Google Scholar] [CrossRef]
- Garg, S.; Ahammed, M.M.; Shaikh, I. Heavy Metal Fractionation in Aerobic and Anaerobic Sewage Sludge. In Current Trends in Civil Engineering; Thomas, J., Jayalekshmi, B.R., Nagarajan, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 11–19. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Sarkar, S.D.; Das, B.K.; Sahoo, B.K.; Das, A.; Nag, S.K.; Manna, R.K.; Behera, B.K.; Samanta, S. Occurrence, fate and removal of microplastics as heavy metal vector in natural wastewater treatment wetland system. Water Res. 2021, 192, 116853. [Google Scholar] [CrossRef]
- Chen, M.; Oshita, K.; Mahzoun, Y.; Takaoka, M.; Fukutani, S.; Shiota, K. Survey of elemental composition in dewatered sludge in Japan. Sci. Total Environ. 2021, 752, 141857. [Google Scholar] [CrossRef] [PubMed]
- da Silva Oliveira, A.; Bocio, A.; Trevilato, T.M.B.; Takayanagui, A.M.M.; Domingo, J.L.; Segura-Muñoz, S.I. Heavy metals in untreated/treated urban effluent and sludge from a biological wastewater treatment plant. Environ. Sci. Pollut. Res. Int. 2007, 14, 483–489. [Google Scholar] [CrossRef]
- Barraoui, D.; Blais, J.-F.; Labrecque, M. Cleanup of sewage sludge spiked with Cd, Cu, and Zn: Sludge quality and distribution of metals in the “soil-plant-water” system. Chemosphere 2021, 267, 129223. [Google Scholar] [CrossRef] [PubMed]
- Pratush, A.; Kumar, A.; Hu, Z. Adverse effect of heavy metals (As, Pb, Hg, and Cr) on health and their bioremediation strategies: A review. Int. Microbiol. 2018, 21, 97–106. [Google Scholar] [CrossRef]
- Mahurpawar, M. Effects of heavy metals on human health. Int. J. Res. Granthaalayah 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Martin, S.; Griswold, W. Human health effects of heavy metals. Environ. Sci. Technol. 2009, 15, 1–6. [Google Scholar]
- Kumar, M.; Puri, A. A review of permissible limits of drinking water. Indian J. Occup. Environ. Med. 2012, 16, 40. [Google Scholar] [PubMed] [Green Version]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef]
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.; Jayasumana, C.; De Silva, P.M.C. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef]
- Oskarsson, A. Barium. In Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2015; pp. 625–634. [Google Scholar]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Du, B.; Zhou, J.; Lu, B.; Zhang, C.; Li, D.; Zhou, J.; Jiao, S.; Zhao, K.; Zhang, H. Environmental and human health risks from cadmium exposure near an active lead-zinc mine and a copper smelter, China. Sci. Total Environ. 2020, 720, 137585. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Ha, E.; Basu, N.; Bose-O’Reilly, S.; Dórea, J.G.; McSorley, E.; Sakamoto, M.; Chan, H.M. Current progress on understanding the impact of mercury on human health. Environ. Res. 2017, 152, 419–433. [Google Scholar] [CrossRef] [Green Version]
- Banu, A.N.; Kudesia, N.; Raut, A.; Pakrudheen, I.; Wahengbam, J. Toxicity, bioaccumulation, and transformation of silver nanoparticles in aqua biota: A review. Environ. Chem. Lett. 2021, 19, 4275–4296. [Google Scholar] [CrossRef]
- Panyala, N.R.; Peña-Méndez, E.M.; Havel, J. Silver or silver nanoparticles: A hazardous threat to the environment and human health? J. Appl. Biomed. 2008, 6, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.; Rezania, S.; Radwan, N.; Alam, J. Chromium contamination and effect on environmental health and its remediation: A sustainable approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef]
- Swanson, C.A. Iron intake and regulation: Implications for iron deficiency and iron overload. Alcohol 2003, 30, 99–102. [Google Scholar] [CrossRef]
- Chen, P.; Culbreth, M.; Aschner, M. Exposure, epidemiology, and mechanism of the environmental toxicant manganese. Environ. Sci. Pollut. Res. 2016, 23, 13802–13810. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of heavy metals from industrial wastewaters: A review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Khandegar, V.; Saroha, A.K. Electrocoagulation for the treatment of textile industry effluent–A review. J. Environ. Manag. 2013, 128, 949–963. [Google Scholar] [CrossRef]
- Gomes, J.A.; Daida, P.; Kesmez, M.; Weir, M.; Moreno, H.; Parga, J.R.; Irwin, G.; McWhinney, H.; Grady, T.; Peterson, E. Arsenic removal by electrocoagulation using combined Al–Fe electrode system and characterization of products. J. Hazard. Mater. 2007, 139, 220–231. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.; Taghipour, F.; Jamshidi-Zanjani, A. A review on industrial wastewater treatment via electrocoagulation processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Kobya, M.; Demirbas, E.; Dedeli, A.; Sensoy, M. Treatment of rinse water from zinc phosphate coating by batch and continuous electrocoagulation processes. J. Hazard. Mater. 2010, 173, 326–334. [Google Scholar] [CrossRef]
- Gu, J.-n.; Liang, J.; Chen, C.; Li, K.; Zhou, W.; Jia, J.; Sun, T. Treatment of real deplating wastewater through an environmental friendly precipitation-electrodeposition-oxidation process: Recovery of silver and copper and reuse of wastewater. Sep. Purif. Technol. 2020, 248, 117082. [Google Scholar] [CrossRef]
- Stando, G.; Hannula, P.-M.; Kumanek, B.; Lundström, M.; Janas, D. Copper recovery from industrial wastewater-Synergistic electrodeposition onto nanocarbon materials. Water Resour. Indus. 2021, 26, 100156. [Google Scholar] [CrossRef]
- Wulan, D.R.; Hariyadi, H.R. Effect of Electrodeposition Reactor Type on Nickel Recovery from Electroplating Wastewater. Procedia Chem. 2015, 16, 155–163. [Google Scholar]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Mishra, N.S.; Reddy, R.; Kuila, A.; Rani, A.; Mukherjee, P.; Nawaz, A.; Pichiah, S. A review on advanced oxidation processes for effective water treatment. Curr. World Environ. 2017, 12, 470. [Google Scholar] [CrossRef]
- Zodi, S.; Potier, O.; Lapicque, F.; Leclerc, J.-P. Treatment of the textile wastewaters by electrocoagulation: Effect of operating parameters on the sludge settling characteristics. Sep. Purif. Technol. 2009, 69, 29–36. [Google Scholar] [CrossRef]
- Ehsani, H.; Mehrdadi, N.; Asadollahfardi, G.; Bidhendi, G.N.; Azarian, G. A new combined electrocoagulation-electroflotation process for pretreatment of synthetic and real Moquette-manufacturing industry wastewater: Optimization of operating conditions. J. Environ. Chem. Eng. 2020, 8, 104263. [Google Scholar] [CrossRef]
- Akarsu, C.; Deniz, F. Electrocoagulation/electroflotation process for removal of organics and microplastics in laundry wastewater. CLEAN–Soil Air Water 2021, 49, 2000146. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X. Chemical precipitation of heavy metals from wastewater by using the synthetical magnesium hydroxy carbonate. Water Sci. Technol. 2020, 81, 1130–1136. [Google Scholar] [CrossRef]
- Stec, M.; Jagustyn, B.; Słowik, K.; Ściążko, M.; Iluk, T. Influence of high chloride concentration on pH control in hydroxide precipitation of heavy metals. J. Sustain. Met. 2020, 6, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Prokkola, H.; Nurmesniemi, E.-T.; Lassi, U. Removal of Metals by Sulphide Precipitation Using Na2S and HS−-Solution. ChemEngineering 2020, 4, 51. [Google Scholar] [CrossRef]
- Qian, J.; Zhu, X.; Tao, Y.; Zhou, Y.; He, X.; Li, D. Promotion of Ni2+ removal by masking toxicity to sulfate-reducing bacteria: Addition of citrate. Int. J. Mol. Sci. 2015, 16, 7932–7943. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.-Y.; Lee, J.-U.; Moon, S.-H.; Kim, K.-W. Competitive adsorption characteristics of Co2+, Ni2+, and Cr3+ by IRN-77 cation exchange resin in synthesized wastewater. Chemosphere 2004, 56, 141–147. [Google Scholar] [CrossRef]
- Elgarahy, A.; Elwakeel, K.; Mohammad, S.; Elshoubaky, G. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Wang, L.K.; Hung, Y.-T.; Shammas, N.K. Physicochemical Treatment Processes; Humana Press: Totowa, NJ, USA, 2005; Volume 3. [Google Scholar]
- Da̧browski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef]
- Tran, A.T.; Pham, T.T.; Nguyen, Q.H.; Hoang, N.T.; Bui, D.T.; Nguyen, M.T.; Nguyen, M.K.; Van der Bruggen, B. From waste disposal to valuable material: Sulfonating polystyrene waste for heavy metal removal. J. Environ. Chem. Eng. 2020, 8, 104302. [Google Scholar] [CrossRef]
- Rengaraj, S.; Yeon, K.-H.; Moon, S.-H. Removal of chromium from water and wastewater by ion exchange resins. J. Hazard. Mater. 2001, 87, 273–287. [Google Scholar] [CrossRef]
- Pandey, S.; Do, J.Y.; Kim, J.; Kang, M. Fast and highly efficient removal of dye from aqueous solution using natural locust bean gum based hydrogels as adsorbent. Int. J. Biol. Macromol. 2020, 143, 60–75. [Google Scholar] [CrossRef]
- Pandey, S.; Tiwari, S. Facile approach to synthesize chitosan based composite—characterization and cadmium(II) ion adsorption studies. Carbohydr. Polym. 2015, 134, 646–656. [Google Scholar] [CrossRef]
- Lakherwal, D. Adsorption of heavy metals: A review. Int. J. Environ. Res. Dev. 2014, 4, 41–48. [Google Scholar]
- Soliman, N.; Moustafa, A. Industrial solid waste for heavy metals adsorption features and challenges; a review. J. Mater. Res. Technol. 2020, 9, 10235–10253. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Cheng, C.K.; Luque, R.; Thomas, S.; Banh, T.L.; Nguyen, X.P. Heavy metal removal by biomass-derived carbon nanotubes as a greener environmental remediation: A comprehensive review. Chemosphere 2021, 287, 131959. [Google Scholar] [CrossRef] [PubMed]
- Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003, 216, 298–312. [Google Scholar] [CrossRef]
- Margeta, K.; Logar, N.Z.; Šiljeg, M.; Farkaš, A. Natural zeolites in water treatment–how effective is their use. Water Treat. 2013, 5, 81–112. [Google Scholar]
- Reza, M.S.; Yun, C.S.; Afroze, S.; Radenahmad, N.; Bakar, M.S.A.; Saidur, R.; Taweekun, J.; Azad, A.K. Preparation of activated carbon from biomass and its’ applications in water and gas purification, a review. Arab J. Basic Appl. Sci. 2020, 27, 208–238. [Google Scholar] [CrossRef]
- Abdel-Halim, S.; Shehata, A.; El-Shahat, M. Removal of lead ions from industrial waste water by different types of natural materials. Water Res. 2003, 37, 1678–1683. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Jiang, Z.; Shi, M.; Sheng, R.; Liu, Z.; Zhang, S.; Cao, Y.; Wei, T.; Fan, Z. Large-surface-area activated carbon with high density by electrostatic densification for supercapacitor electrodes. Carbon 2021, 175, 281–288. [Google Scholar] [CrossRef]
- Erdoğan, S.; Önal, Y.; Akmil-Başar, C.; Bilmez-Erdemoğlu, S.; Sarıcı-Özdemir, Ç.; Köseoğlu, E.; Icduygu, G. Optimization of nickel adsorption from aqueous solution by using activated carbon prepared from waste apricot by chemical activation. Appl. Surf. Sci. 2005, 252, 1324–1331. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Kabbashi, N.A.; Atieh, M.A.; Al-Mamun, A.; Mirghami, M.E.; Alam, M.; Yahya, N. Kinetic adsorption of application of carbon nanotubes for Pb(II) removal from aqueous solution. J. Environ. Sci. 2009, 21, 539–544. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Xia, B.; Du, Q.; Zhang, P.; Wang, D.; Wang, Z.; Xia, Y. Removal of copper from aqueous solution by carbon nanotube/calcium alginate composites. J. Hazard. Mater. 2010, 177, 876–880. [Google Scholar] [CrossRef]
- Šćiban, M.; Radetić, B.; Kevrešan, Ž.; Klašnja, M. Adsorption of heavy metals from electroplating wastewater by wood sawdust. Bioresour. Technol. 2007, 98, 402–409. [Google Scholar] [CrossRef]
- Sahmoune, M.N.; Yeddou, A.R. Potential of sawdust materials for the removal of dyes and heavy metals: Examination of isotherms and kinetics. Desalin. Water Treat. 2016, 57, 24019–24034. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Zhang, L.; Li, S.; Yuan, Y.; Xiao, X.; Fan, X.; Song, C. Carbon-based membrane materials and applications in water and wastewater treatment: A review. Environ. Chem. Lett. 2021, 19, 1457–1475. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mohammadi, T.; Nikouzad, S.K. A positively charged composite loose nanofiltration membrane for water purification from heavy metals. J. Membr. Sci. 2020, 611, 118205. [Google Scholar] [CrossRef]
- Spoială, A.; Ilie, C.-I.; Ficai, D.; Ficai, A.; Andronescu, E. Chitosan-Based Nanocomposite Polymeric Membranes for Water Purification—A Review. Materials 2021, 14, 2091. [Google Scholar] [CrossRef]
- Das, T.K.; Remanan, S.; Ghosh, S.; Das, N.C. An environment friendly free-standing cellulose membrane derived for catalytic reduction of 4-nitrophenol: A sustainable approach. J. Environ. Chem. Eng. 2021, 9, 104596. [Google Scholar] [CrossRef]
- Rani, S.L.S.; Kumar, R.V. Insights on applications of low-cost ceramic membranes in wastewater treatment: A mini-review. Case Stud. Chem. Environ. Eng. 2021, 4, 100149. [Google Scholar] [CrossRef]
- Murthy, Z.; Chaudhari, L.B. Separation of binary heavy metals from aqueous solutions by nanofiltration and characterization of the membrane using Spiegler–Kedem model. Chem. Eng. J. 2009, 150, 181–187. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Agustina, T.E.; Ang, H.M.; Vareek, V.K. A review of synergistic effect of photocatalysis and ozonation on wastewater treatment. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 264–273. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Puma, G.L.; Ye, J.; Schneider, J.; Bahnemann, D. Photocatalysis: Applications; Royal Society of Chemistry: Croydon, UK, 2016. [Google Scholar]
- Fresno, F.; Portela, R.; Suárez, S.; Coronado, J.M. Photocatalytic materials: Recent achievements and near future trends. J. Mater. Chem. A 2014, 2, 2863–2884. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Feng, C. Application of photocatalytic technology in environmental safety. Procedia Eng. 2012, 45, 993–997. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-González, V.; Obregón, S.; Patrón-Soberano, O.A.; Terashima, C.; Fujishima, A. An approach to the photocatalytic mechanism in the TiO2-nanomaterials microorganism interface for the control of infectious processes. Appl. Catal. B Environ. 2020, 270, 118853. [Google Scholar] [CrossRef]
- Herrmann, J.-M.; Guillard, C.; Pichat, P. Heterogeneous photocatalysis: An emerging technology for water treatment. Catal. Today 1993, 17, 7–20. [Google Scholar] [CrossRef]
- Molinari, R.; Caruso, A.; Poerio, T. Direct benzene conversion to phenol in a hybrid photocatalytic membrane reactor. Catal. Today 2009, 144, 81–86. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barani, M.; Hosseinikhah, S.M.; Rahdar, A.; Farhoudi, L.; Arshad, R.; Cucchiarini, M.; Pandey, S. Nanotechnology in Bladder Cancer: Diagnosis and Treatment. Cancers 2021, 13, 2214. [Google Scholar] [CrossRef] [PubMed]
- Sadegh, H.; Ali, G.A. Potential applications of nanomaterials in wastewater treatment: Nanoadsorbents performance. In Research Anthology on Synthesis, Characterization, and Applications of Nanomaterials; IGI Global: Hershay, PA, USA, 2021; pp. 1230–1240. [Google Scholar]
- Davarazar, M.; Kamali, M.; Lopes, I. Engineered nanomaterials for (waste)water treatment-A scientometric assessment and sustainability aspects. NanoImpact 2021, 22, 100316. [Google Scholar] [CrossRef]
- Cheriyamundath, S.; Vavilala, S.L. Nanotechnology-based wastewater treatment. Water Environ. J. 2021, 35, 123–132. [Google Scholar] [CrossRef]
- Torgal, F.P.; Diamanti, M.V.; Granqvist, C.G.; Pruna, A.; Amirkhanian, S. Nanotechnology in Eco-Efficient Construction; Woodhead Publishing: Duxford, UK, 2013. [Google Scholar]
- Das, T.K.; Ganguly, S.; Bhawal, P.; Remanan, S.; Ghosh, S.; Das, N.C. A facile green synthesis of silver nanoparticles decorated silica nanocomposites using mussel inspired polydopamine chemistry and assessment its catalytic activity. J. Environ. Chem. Eng. 2018, 6, 6989–7001. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Nanoadsorbents for pollutants removal: A review. J. Mol. Liq. 2015, 203, 159–168. [Google Scholar] [CrossRef]
- Singh, S.; Barick, K.; Bahadur, D. Surface engineered magnetic nanoparticles for removal of toxic metal ions and bacterial pathogens. J. Hazard. Mater. 2011, 192, 1539–1547. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Luque, R.; Fihri, A.; Zhu, H.; Bouhrara, M.; Basset, J.-M. Magnetically recoverable nanocatalysts. Chem. Rev. 2011, 111, 3036–3075. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.-M.; Flora, J.R.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Taşar, Ş.; Kaya, F.; Özer, A. Biosorption of lead(II) ions from aqueous solution by peanut shells: Equilibrium, thermodynamic and kinetic studies. J. Environ. Chem. Eng. 2014, 2, 1018–1026. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Dai, G.; Wu, J.; Yan, H. Adsorption characteristics of Pb(II) from aqueous solution onto a natural biosorbent, fallen Cinnamomum camphora leaves. Desalination 2010, 262, 174–182. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y. Investigations of nickel(II) removal from aqueous solutions using tea factory waste. J. Hazard. Mater. 2005, 127, 120–128. [Google Scholar] [CrossRef]
- Weng, C.-H.; Lin, Y.-T.; Hong, D.-Y.; Sharma, Y.C.; Chen, S.-C.; Tripathi, K. Effective removal of copper ions from aqueous solution using base treated black tea waste. Ecol. Eng. 2014, 67, 127–133. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M. Biosorption of total chromium from aqueous solution by red algae (Ceramium virgatum): Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 160, 349–355. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Kirupha, S.D.; Murugesan, A.; Vidhyadevi, T.; Sivanesan, S. Adsorption behavior of nickel(II) onto cashew nut shell: Equilibrium, thermodynamics, kinetics, mechanism and process design. Chem. Eng. J. 2011, 167, 122–131. [Google Scholar] [CrossRef]
- Ferraz, M.A.; Lourençlo, J. The influence of organic matter content of contaminated soils on the leaching rate of heavy metals. Environ. Prog. 2000, 19, 53–58. [Google Scholar] [CrossRef]
- Villaescusa, I.; Fiol, N.; Martínez, M.a.; Miralles, N.; Poch, J.; Serarols, J. Removal of copper and nickel ions from aqueous solutions by grape stalks wastes. Water Res. 2004, 38, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M. Adsorption study of Pb(II), Cu(II) and Zn(II) from simulated acid mine drainage using dairy manure compost. Chem. Eng. J. 2011, 172, 361–368. [Google Scholar] [CrossRef]
- Yang, S.; Wu, Y.; Aierken, A.; Zhang, M.; Fang, P.; Fan, Y.; Ming, Z. Mono/competitive adsorption of Arsenic(III) and Nickel(II) using modified green tea waste. J. Taiwan Inst. Chem. Eng. 2016, 60, 213–221. [Google Scholar] [CrossRef]
- Liu, Z.; Papineau, I.; Mohseni, M.; Peldszus, S.; Bérubé, P.R.; Sauvé, S.; Barbeau, B. Operating Bicarbonate-Form versus Chloride-Form Ion Exchange Resins without Regeneration for Natural Organic Matter Removal. ACS ES & T Water 2021, 1, 1456–1463. [Google Scholar]
- Engel, M.; Pacheco, J.S.L.; Noël, V.; Boye, K.; Fendorf, S. Organic compounds alter the preference and rates of heavy metal adsorption on ferrihydrite. Sci. Total Environ. 2021, 750, 141485. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mulligan, C.N. Natural attenuation processes for remediation of arsenic contaminated soils and groundwater. J. Hazard. Mater. 2006, 138, 459–470. [Google Scholar] [CrossRef]
- Du, Y.; Lian, F.; Zhu, L. Biosorption of divalent Pb, Cd and Zn on aragonite and calcite mollusk shells. Environ. Pollut. 2011, 159, 1763–1768. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments–A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Godiya, C.B.; Liang, M.; Sayed, S.M.; Li, D.; Lu, X. Novel alginate/polyethyleneimine hydrogel adsorbent for cascaded removal and utilization of Cu2+ and Pb2+ ions. J. Environ. Manag. 2019, 232, 829–841. [Google Scholar] [CrossRef]
- Ma, W.; Zong, P.; Cheng, Z.; Wang, B.; Sun, Q. Adsorption and bio-sorption of nickel ions and reuse for 2-chlorophenol catalytic ozonation oxidation degradation from water. J. Hazard. Mater. 2014, 266, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Das, R.; van der Westhuyzen, C.; Maity, A. An efficient selective reduction of nitroarenes catalyzed by reusable silver-adsorbed waste nanocomposite. Appl. Catal. B Environ. 2017, 209, 669–678. [Google Scholar] [CrossRef]
- Godiya, C.B.; Cheng, X.; Deng, G.; Li, D.; Lu, X. Silk fibroin/polyethylenimine functional hydrogel for metal ion adsorption and upcycling utilization. J. Environ. Chem. Eng. 2019, 7, 102806. [Google Scholar] [CrossRef]
- Das, R.; Giri, S.; King Abia, A.L.; Dhonge, B.; Maity, A. Removal of noble metal ions (Ag+) by mercapto group-containing polypyrrole matrix and reusability of its waste material in environmental applications. ACS Sustain. Chem. Eng. 2017, 5, 2711–2724. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, J.; Xiao, Y.; Du, J. Efficient removal of polycyclic aromatic hydrocarbons, dyes, and heavy metal ions by a homopolymer vesicle. ACS Appl. Mater. Interface 2018, 10, 713–722. [Google Scholar] [CrossRef]
- Javed, R.; Shah, L.A.; Sayed, M.; Khan, M.S. Uptake of heavy metal ions from aqueous media by hydrogels and their conversion to nanoparticles for generation of a catalyst system: Two-fold application study. RSC Adv. 2018, 8, 14787–14797. [Google Scholar] [CrossRef] [Green Version]
- Su, Q.; Yang, S.; He, Y.; Qin, Z.; Cui, X. Prepared self-growing supported nickel catalyst by recovering Ni(Ⅱ) from metal wastewater using geopolymer microspheres. J. Hazard. Mater. 2020, 389, 121919. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Bikiaris, D.N.; Kyzas, G.Z. Chitin adsorbents for toxic metals: A review. Int. J. Mol. Sci. 2017, 18, 114. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Bai, Z.; Jiang, H.; Prinsen, P.; Luque, R.; Zhao, S.; Xuan, J. Selective heavy metal removal and water purification by microfluidically-generated chitosan microspheres: Characteristics, modeling and application. J. Hazard. Mater. 2019, 364, 192–205. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, Q.; Yang, W.; Pei, H.; Zhang, L.; Zhang, T.; Hu, N.; Suo, Y.; Wang, J. Adsorptive catalysis of hierarchical porous heteroatom-doped biomass: From recovered heavy metal to efficient pollutant decontamination. J. Mater. Chem. A 2018, 6, 16690–16698. [Google Scholar] [CrossRef]
- Kakaei, S.; Khameneh, E.; Hosseini, M.; Moharreri, M. A modified ionic liquid clay to remove heavy metals from water: Investigating its catalytic activity. Int. J. Environ. Sci. Technol. 2020, 17, 2043–2058. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-J.; Zeng, H.-Y.; Xu, S.; Chen, C.-R.; Zhang, Z.-q.; Du, J.-Z. Metal oxides as dual-functional adsorbents/catalysts for Cu2+/Cr(VI) adsorption and methyl orange oxidation catalysis. J. Taiwan Inst. Chem. Eng. 2016, 60, 414–422. [Google Scholar] [CrossRef]
- Ivanets, A.; Roshchina, M.; Srivastava, V.; Prozorovich, V.; Dontsova, T.; Nahirniak, S.; Pankov, V.; Hosseini-Bandegharaei, A.; Tran, H.N.; Sillanpää, M. Effect of metal ions adsorption on the efficiency of methylene blue degradation onto MgFe2O4 as Fenton-like catalysts. Colloid Surf. A 2019, 571, 17–26. [Google Scholar] [CrossRef]
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P.K. Integrated remediation processes toward heavy metal removal/recovery from various environments-a review. Front. Environ. Sci. 2019, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Weng, C.; Zheng, J.; Peng, X.; Zhang, J.; Lin, Z. Emerging investigator series: Treatment and recycling of heavy metals from nanosludge. Environ. Sci. Nano 2019, 6, 1657–1673. [Google Scholar] [CrossRef]
- Bassyouni, M.; Mansi, A.; Elgabry, A.; Ibrahim, B.A.; Kassem, O.A.; Alhebeshy, R. Utilization of carbon nanotubes in removal of heavy metals from wastewater: A review of the CNTs’ potential and current challenges. Appl. Phys. A 2020, 126, 1–33. [Google Scholar] [CrossRef]
- Gautam, R.K.; Chattopadhyaya, M.C.; Sharma, S.K. Biosorption of heavy metals: Recent trends and challenges. Wastewater Reuse Manag. 2013, 305–322. [Google Scholar] [CrossRef]
- Mao, S.; Ga, M. Functional organoclays for removal of heavy metal ions from water: A review. J. Mol. Liq. 2021, 334, 116143. [Google Scholar] [CrossRef]
- Kumar, V.; Chawla, M.; Cavallo, L.; Wani, A.B.; Manhas, A.; Kaur, S.; Poater, A.; Chadar, H.; Upadhyay, N. Complexation of trichlorosalicylic acid with alkaline and first row transition metals as a switch for their antibacterial activity. Inorg. Chim. Acta 2018, 469, 379–386. [Google Scholar] [CrossRef] [Green Version]





| Sl.No. | Heavy Metals | Name of the Organization | Regulatory Limits |
|---|---|---|---|
| 1. | Silver (Ag) | EPA | Drinking water: not exceed 0.10 ppb |
| Occupational Safety and Health Administration (OSHA) | 0.01 mg/m3 in workplace air | ||
| 2. | Arsenic (As) | EPA | Drinking water: 0.01 parts per million (ppm) |
| OSHA | 10.0 µg/m3 in workplace | ||
| 3. | Barium (Ba) | EPA | Drinking water: 2.0 ppm |
| OSHA | 0.5 mg/m3 in workplace for soluble barium compounds | ||
| 4. | Cadmium (Cd) | EPA | Drinking water: 0.005 ppm |
| OSHA | 5.0 µg/m3 in workplace | ||
| Food and Drug Administration (FDA) | Bottled drinking water: 5 parts per billion (ppb) | ||
| 5. | Cobalt (Co) | EPA | Drinking water:1–2 ppb |
| OSHA | 0.1 mg/m3 in workplace air | ||
| 6. | Chromium (Cr) | EPA | Drinking water: 0.1 ppm |
| OSHA | 0.0005–1.0 mg/m3 in workplace depending on the compounds | ||
| FDA | Bottled drinking water: 1 ppm | ||
| 7. | Copper (Cu) | EPA | Drinking water:1.5 mg/L |
| World Health Organization (WHO) | 2 mg/L | ||
| 8. | Iron (Fe) | EPA | Drinking water: 0.3 mg/L |
| WHO | Drinking water:0.1 mg/L | ||
| 9. | Mercury (Hg) | EPA | Drinking water:2 ppb |
| OSHA | 0.1 mg/m3 in workplace for organic Hg and 0.05 mg/m3 in workplace for metallic Hg vapour | ||
| FDA | 1 part of methylmercury in a million parts of seafood | ||
| 10. | Manganese (Mn) | WHO | 0.4 mg/L |
| EPA | Drinking water: 0.05 mg/L | ||
| 11. | Nickel (Ni) | WHO | Drinking water: 70 µg/L |
| EU (European Union) | Drinking water: 20 µg/L | ||
| 12. | Lead (Pb) | EPA | Drinking water: 15 ppb and 0.15 µg/m3 in air. |
| 13. | Selenium (Se) | EPA | Drinking water: 50 ppb |
| OSHA | 0.2 mg/m3 in workplace air | ||
| 14. | Zinc (Zn) | EPA | Drinking water: 5 mg/L |
| OSHA | 1 mg/m3 for zinc chloride fumes and 5 mg/m3 for zinc oxide (Dust form) in workplace air |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, T.K.; Poater, A. Review on the Use of Heavy Metal Deposits from Water Treatment Waste towards Catalytic Chemical Syntheses. Int. J. Mol. Sci. 2021, 22, 13383. https://doi.org/10.3390/ijms222413383
Das TK, Poater A. Review on the Use of Heavy Metal Deposits from Water Treatment Waste towards Catalytic Chemical Syntheses. International Journal of Molecular Sciences. 2021; 22(24):13383. https://doi.org/10.3390/ijms222413383
Chicago/Turabian StyleDas, Tushar Kanti, and Albert Poater. 2021. "Review on the Use of Heavy Metal Deposits from Water Treatment Waste towards Catalytic Chemical Syntheses" International Journal of Molecular Sciences 22, no. 24: 13383. https://doi.org/10.3390/ijms222413383