Isoprenoid Derivatives of Lysophosphatidylcholines Enhance Insulin and GLP-1 Secretion through Lipid-Binding GPCRs
Abstract
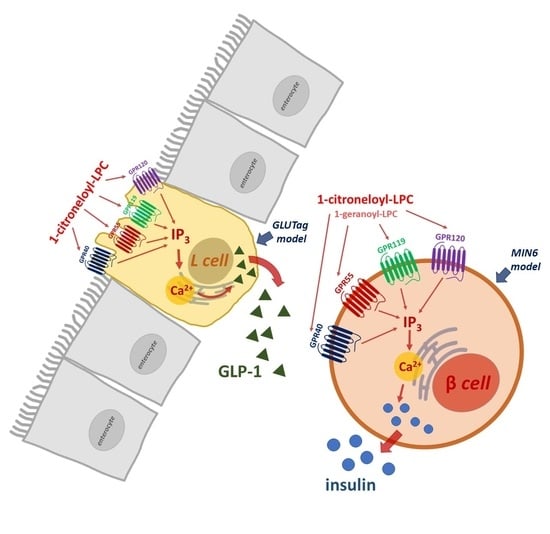
:1. Introduction
2. Results
2.1. Determination of MIN6 Metabolic Activity and Proliferation under the Influence of Isoprenoid Derivatives of LPCs
2.2. The Influence of Isoprenoid Derivatives of LPCs on GSIS and Intracellular Ca2+ Mobilization in MIN6 Cells
2.3. The Role of GPR40, GPR55, GPR119, and GPR120 in GSIS and Intracellular Ca2+ Mobilization Evoked by Isoprenoid Derivatives of LPCs in MIN6 Cells
2.4. The Influence of Isoprenoid Derivatives of LPCs on GLP-1 Secretion in GLUTag Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. MIN6 Cell Culture
4.3. GLUTag Cell Culture
4.4. Cell Viability
4.5. Glucose-Stimulated Insulin Secretion (GSIS)
4.6. Glucagon-Like Peptide 1 (GLP-1) Secretion
4.7. Intracellular Calcium ([Ca2+]i) Mobilization
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, P.-J.; Pope, E.; Zhou, F.L. Comorbidity Type and Health Care Costs in Type 2 Diabetes: A Retrospective Claims Database Analysis. Diabetes Ther. 2018, 9, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Bodnaruc, A.M.; Prud’Homme, D.; Blanchet, R.; Giroux, I. Nutritional modulation of endogenous glucagon-like peptide-1 secretion: A review. Nutr. Metab. 2016, 13, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauck, M.A.; Friedrich, N. Do GLP-1-Based Therapies Increase Cancer Risk? Diabetes Care 2013, 36, S245–S252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadkarni, P.; Chepurny, O.G.; Holz, G.G. Regulation of Glucose Homeostasis by GLP-1. Prog. Mol. Biol. Transl. Sci. 2014, 121, 23–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, R.; Dalziel, J.E. G Protein-Coupled Receptors in Taste Physiology and Pharmacology. Front. Pharmacol. 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Röthe, J.; Kraft, R.; Schöneberg, T.; Thor, D. Exploring G Protein-Coupled Receptor Signaling in Primary Pancreatic Islets. Biol. Proced. Online 2020, 22, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Gendaszewska-Darmach, E.; Drzazga, A.; Koziołkiewicz, M. Targeting GPCRs Activated by Fatty Acid-Derived Lipids in Type 2 Diabetes. Trends Mol. Med. 2019, 25, 915–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soga, T.; Ohishi, T.; Matsui, T.; Saito, T.; Matsumoto, M.; Takasaki, J.; Matsumoto, S.-I.; Kamohara, M.; Hiyama, H.; Yoshida, S.; et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 2005, 326, 744–751. [Google Scholar] [CrossRef]
- Heimerl, S.; Fischer, M.; Baessler, A.; Liebisch, G.; Sigruener, A.; Wallner, S.; Schmitz, G. Alterations of Plasma Lysophosphatidylcholine Species in Obesity and Weight Loss. PLoS ONE 2014, 9, e111348. [Google Scholar] [CrossRef] [Green Version]
- Yea, K.; Kim, J.; Yoon, J.H.; Kwon, T.; Kim, J.H.; Lee, B.D.; Lee, H.-J.; Lee, S.J.; Lee, T.G.; Baek, M.-C.; et al. Lysophosphatidylcholine Activates Adipocyte Glucose Uptake and Lowers Blood Glucose Levels in Murine Models of Diabetes. J. Biol. Chem. 2009, 284, 33833–33840. [Google Scholar] [CrossRef] [Green Version]
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.C.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615. [Google Scholar] [CrossRef]
- Drzazga, A.; Cichońska, E.; Koziołkiewicz, M.; Gendaszewska-Darmach, E. Formation of βTC3 and MIN6 Pseudoislets Changes the Expression Pattern of Gpr40, Gpr55, and Gpr119 Receptors and Improves Lysophosphatidylcholines-Potentiated Glucose-Stimulated Insulin Secretion. Cells 2020, 9, 2062. [Google Scholar] [CrossRef]
- Drzazga, A.; Kristinsson, H.; Sałaga, M.; Zatorski, H.; Koziołkiewicz, M.; Gendaszewska-Darmach, E.; Bergsten, P. Lysophosphatidylcholine and its phosphorothioate analogues potentiate insulin secretion via GPR40 (FFAR1), GPR55 and GPR119 receptors in a different manner. Mol. Cell. Endocrinol. 2018, 472, 117–125. [Google Scholar] [CrossRef]
- Drzazga, A.; Okulus, M.; Rychlicka, M.; Biegała, Ł.; Gliszczyńska, A.; Gendaszewska-Darmach, E. Lysophosphatidylcholine Containing Anisic Acid Is Able to Stimulate Insulin Secretion Targeting G Protein Coupled Receptors. Nutrients 2020, 12, 1173. [Google Scholar] [CrossRef]
- Valdes, M.; Calzada, F.; Mendieta-Wejebe, J. Structure–Activity Relationship Study of Acyclic Terpenes in Blood Glucose Levels: Potential α-Glucosidase and Sodium Glucose Cotransporter (SGLT-1) Inhibitors. Molecules 2019, 24, 4020. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, W.; Cheng, S.; Cao, D.; Parent, M. Isoprenoids and Related Pharmacological Interventions: Potential Application in Alzheimer’s Disease. Mol. Neurobiol. 2012, 46, 64–77. [Google Scholar] [CrossRef]
- Kowluru, A.; Kowluru, R.A. Protein prenylation in islet β-cell function in health and diabetes: Putting the pieces of the puzzle together. Biochem. Pharmacol. 2015, 98, 363–370. [Google Scholar] [CrossRef]
- Idevall-Hagren, O.; Tengholm, A. Metabolic regulation of calcium signaling in beta cells. Semin. Cell Dev. Biol. 2020, 103, 20–30. [Google Scholar] [CrossRef]
- Frasch, S.C.; Zemski-Berry, K.; Murphy, R.C.; Borregaard, N.; Henson, P.M.; Bratton, D.L. Lysophospholipids of Different Classes Mobilize Neutrophil Secretory Vesicles and Induce Redundant Signaling through G2A. J. Immunol. 2007, 178, 6540–6548. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xie, T.; Zhang, D.; Leung, P.S. GPR120 protects lipotoxicity-induced pancreatic β-cell dysfunction through regulation of PDX1 expression and inhibition of islet inflammation. Clin. Sci. 2019, 133, 101–116. [Google Scholar] [CrossRef]
- Bai, L.; Meredith, G.; Tuch, E.B. Glucagon-like peptide-1 enhances production of insulin in insulin-producing cells derived from mouse embryonic stem cells. J. Endocrinol. 2005, 186, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, M.; Jehle, D.R.; Fussgaenger, R.D.; Fehmann, H.C.; Jehle, P.M. Glucagon-like peptide-1 improves insulin and proinsulin binding on RINm5F cells and human monocytes. Am. J. Physiol. Metab. 2000, 279, E88–E94. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Shi, S.; Lao, X.; Zhang, J.; Tan, S.; Wu, Z.; Huang, J. A novel GLP-1 analog, a dimer of GLP-1 via covalent linkage by a lysine, prolongs the action of GLP-1 in the treatment of type 2 diabetes. Peptides 2017, 88, 46–54. [Google Scholar] [CrossRef]
- Samtleben, S.; Jaepel, J.; Fecher, C.; Andreska, T.; Rehberg, M.; Blum, R. Direct Imaging of ER Calcium with Targeted-Esterase Induced Dye Loading (TED). J. Vis. Exp. 2013, 50317, e50317. [Google Scholar] [CrossRef] [PubMed]
- Chepurny, O.G.; Bertinetti, D.; Diskar, M.; Leech, C.A.; Afshari, P.; Tsalkova, T.; Cheng, X.; Schwede, F.; Genieser, H.-G.; Herberg, F.W.; et al. Stimulation of proglucagon gene expression by human GPR119 in enteroendocrine L-cell line GLUTag. Mol. Endocrinol. 2013, 27, 1267–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamakura, R.; Raza, G.S.; Prasannan, A.; Walkowiak, J.; Herzig, K.-H. Dipeptidyl peptidase-4 and GLP-1 interplay in STC-1 and GLUTag cell lines. Peptides 2020, 134, 170419. [Google Scholar] [CrossRef] [PubMed]
- Arifin, S.A.; Paternoster, S.; Carlessi, R.; Casari, I.; Ekberg, J.H.; Maffucci, T.; Newsholme, P.; Rosenkilde, M.M.; Falasca, M. Oleoyl-lysophosphatidylinositol enhances glucagon-like peptide-1 secretion from enteroendocrine L-cells through GPR119. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2018, 1863, 1132–1141. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Deng, X.; Yin, F. Geniposide decreases the level of A 1-42 in the hippocampus of streptozotocin-induced diabetic rats. Acta Biochim. Biophys. Sin. 2013, 45, 787–791. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.Y.; Hao, Y.N.; Yin, F.; Zhang, Y.L.; Liu, J.H. Geniposide accelerates proteasome degradation of Txnip to inhibit insulin secretion in pancreatic β-cells. J. Endocrinol. Investig. 2016, 40, 505–512. [Google Scholar] [CrossRef]
- Liu, C.; Hao, Y.; Yin, F.; Zhang, Y.; Liu, J. Geniposide protects pancreatic β cells from high glucose-mediated injury by activation of AMP-activated protein kinase. Cell Biol. Int. 2017, 41, 544–554. [Google Scholar] [CrossRef]
- Soundharrajan, I.; Kim, D.H.; Srisesharam, S.; Kuppusamy, P.; Choi, K.C. R-Limonene Enhances Differentiation and 2-Deoxy-D-Glucose Uptake in 3T3-L1 Preadipocytes by Activating the Akt Signaling Pathway. Evid. Based Complement. Altern. Med. 2018, 2018, 4573254. [Google Scholar] [CrossRef]
- Tan, X.; Chua, K.; Ram, M.R.; Kuppusamy, U. Monoterpenes: Novel insights into their biological effects and roles on glucose uptake and lipid metabolism in 3T3-L1 adipocytes. Food Chem. 2016, 196, 242–250. [Google Scholar] [CrossRef]
- Srinivasan, S.; Muruganathan, U. Antidiabetic efficacy of citronellol, a citrus monoterpene by ameliorating the hepatic key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Chem. Interact. 2016, 250, 38–46. [Google Scholar] [CrossRef]
- Babukumar, S.; Vinothkumar, V.; Sankaranarayanan, C.; Srinivasan, S. Geraniol, a natural monoterpene, ameliorates hyperglycemia by attenuating the key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Pharm. Biol. 2017, 55, 1442–1449. [Google Scholar] [CrossRef] [Green Version]
- Bacanlı, M.; Anlar, H.G.; Aydın, S.; Çal, T.; Arı, N.; Bucurgat, Ü.Ü.; Başaran, A.A.; Başaran, N. D-limonene ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2017, 110, 434–442. [Google Scholar] [CrossRef]
- Murali, R.; Saravanan, R. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomed. Prev. Nutr. 2012, 2, 269–275. [Google Scholar] [CrossRef]
- Pedersen, P.J.; Christensen, M.S.; Ruysschaert, T.; Linderoth, L.; Andresen, T.L.; Melander, F.; Mouritsen, O.G.; Madsen, R.; Clausen, M.H. Synthesis and Biophysical Characterization of Chlorambucil Anticancer Ether Lipid Prodrugs. J. Med. Chem. 2009, 52, 3408–3415. [Google Scholar] [CrossRef]
- Liu, H.; Bolleddula, J.; Nichols, A.; Tang, L.; Zhao, Z.; Prakash, C. Metabolism of bioconjugate therapeutics: Why, when, and how? Drug Metab. Rev. 2020, 52, 66–124. [Google Scholar] [CrossRef]
- Gliszczyńska, A.; Niezgoda, N.; Gładkowski, W.; Czarnecka, M.; Świtalska, M.; Wietrzyk, J. Synthesis and Biological Evaluation of Novel Phosphatidylcholine Analogues Containing Monoterpene Acids as Potent Antiproliferative Agents. PLoS ONE 2016, 11, e0157278. [Google Scholar] [CrossRef]
- Gliszczyńska, A.; Niezgoda, N.; Gładkowski, W.; Świtalska, M.; Wietrzyk, J. Isoprenoid-phospholipid conjugates as potential therapeutic agents: Synthesis, characterization and antiproliferative studies. PLoS ONE 2017, 12, e0172238. [Google Scholar] [CrossRef]
- Rytczak, P.; Drzazga, A.; Gendaszewska-Darmach, E.; Okruszek, A. The chemical synthesis and cytotoxicity of new sulfur analogues of 2-methoxy-lysophosphatidylcholine. Bioorg. Med. Chem. Lett. 2013, 23, 6794–6798. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Gendaszewska-Darmach, E.; Drzazga, A. Sulfur- and Acyl Chain-Dependent Influence of 2-Methoxy-Lysophosphatidylcholine Analogues on β Pancreatic Cells. Curr. Top. Med. Chem. 2015, 15, 2395–2405. [Google Scholar] [CrossRef]
- Müller, T.; Finan, B.; Bloom, S.; D’Alessio, D.; Drucker, D.; Flatt, P.; Fritsche, A.; Gribble, F.; Grill, H.; Habener, J.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Dankner, R.; Chetrit, A.; Shanik, M.H.; Raz, I.; Roth, J. Basal-State Hyperinsulinemia in Healthy Normoglycemic Adults Is Predictive of Type 2 Diabetes Over a 24-Year Follow-Up: A preliminary report. Diabetes Care 2009, 32, 1464–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Nakamura, H.; Sato, H.; Matsuda, H.; Takada, K.; Tsuji, T. Four Plasma Glucose and Insulin Responses to a 75 g OGTT in Healthy Young Japanese Women. J. Diabetes Res. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, S.; Okamoto, Y.; Kawabata, J. Radical scavenging mechanism of phenol carboxylic acids: Reaction of protocatechuic esters. BioFactors 2004, 21, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hao, Y.; Yin, F.; Liu, J. Geniposide Balances the Redox Signaling to Mediate Glucose-Stimulated Insulin Secretion in Pancreatic β-Cells. Diabetes Metab. Syndr. Obesity Targets Ther. 2020, 13, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Dhyani, V.; Gare, S.; Gupta, R.K.; Swain, S.; Venkatesh, K.; Giri, L. GPCR mediated control of calcium dynamics: A systems perspective. Cell. Signal. 2020, 74, 109717. [Google Scholar] [CrossRef]
- Sassmann, A.; Gier, B.; Gröne, H.-J.; Drews, G.; Offermanns, S.; Wettschureck, N. The Gq/G11-mediated signaling pathway is critical for autocrine potentiation of insulin secretion in mice. J. Clin. Investig. 2010, 120, 2184–2193. [Google Scholar] [CrossRef] [Green Version]
- Reimann, F.; Williams, L.; Xavier, G.D.S.; Rutter, G.A.; Gribble, F.M. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetology 2004, 47, 1592–1601. [Google Scholar] [CrossRef] [Green Version]
- Stafford, R.E.; Fanni, T.; Dennis, A.E. Interfacial properties and critical micelle concentration of lysophospholipids. Biochemistry 1989, 28, 5113–5120. [Google Scholar] [CrossRef]
- McClure, K.F.; Darout, E.; Guimarães, C.R.W.; DeNinno, M.P.; Mascitti, V.; Munchhof, M.J.; Robinson, R.P.; Kohrt, J.; Harris, A.R.; Moore, D.E.; et al. Activation of the G-Protein-Coupled Receptor 119: A Conformation-Based Hypothesis for Understanding Agonist Response. J. Med. Chem. 2011, 54, 1948–1952. [Google Scholar] [CrossRef]
- Miyazaki, J.-I.; Araki, K.; Yamato, E.; Ikegami, H.; Asano, T.; Shibasaki, Y.; Oka, Y.; Yamamura, K.-I. Establishment of a Pancreatic β Cell Line That Retains Glucose-Inducible Insulin Secretion: Special Reference to Expression of Glucose Transporter Isoforms*. Endocrinology 1990, 127, 126–132. [Google Scholar] [CrossRef]
- Drucker, D.J.; Jin, T.; Asa, S.; Young, A.T.; Brubaker, P.L. Activation of proglucagon gene transcription by protein kinase-A in a novel mouse enteroendocrine cell line. Mol. Endocrinol. 1994, 8, 1646–1655. [Google Scholar] [CrossRef] [Green Version]
- Bergsten, P.; Hellman, B. Glucose-Induced Amplitude Regulation of Pulsatile Insulin Secretion from Individual Pancreatic Islets. Diabetes 1993, 42, 670–674. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drzazga, A.; Kamińska, D.; Gliszczyńska, A.; Gendaszewska-Darmach, E. Isoprenoid Derivatives of Lysophosphatidylcholines Enhance Insulin and GLP-1 Secretion through Lipid-Binding GPCRs. Int. J. Mol. Sci. 2021, 22, 5748. https://doi.org/10.3390/ijms22115748
Drzazga A, Kamińska D, Gliszczyńska A, Gendaszewska-Darmach E. Isoprenoid Derivatives of Lysophosphatidylcholines Enhance Insulin and GLP-1 Secretion through Lipid-Binding GPCRs. International Journal of Molecular Sciences. 2021; 22(11):5748. https://doi.org/10.3390/ijms22115748
Chicago/Turabian StyleDrzazga, Anna, Daria Kamińska, Anna Gliszczyńska, and Edyta Gendaszewska-Darmach. 2021. "Isoprenoid Derivatives of Lysophosphatidylcholines Enhance Insulin and GLP-1 Secretion through Lipid-Binding GPCRs" International Journal of Molecular Sciences 22, no. 11: 5748. https://doi.org/10.3390/ijms22115748