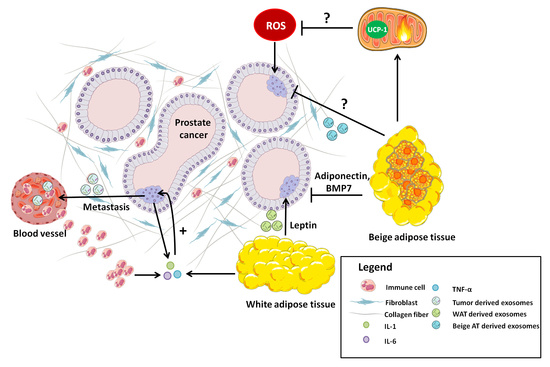
Emerging Roles for Browning of White Adipose Tissue in Prostate Cancer Malignant Behaviour
Abstract
:1. White Adipose Tissue (WAT)
2. Brown Adipose Tissue (BAT)
| Characteristic | White | Brown | Beige |
|---|---|---|---|
| Morphology Shape | Spherical | Polygonal | Spherical |
| Cell Size | Variable, large | Small | Variable |
| Lipid Droplet | Single large LD | Multiple small | Multiple variable |
| Mitochondria | + | +++ | ++ (upon stimulation) |
| Development | From Myf 5− precursors | From Myf 5+ precursors | From Myf 5− precursors |
| Location | Subcutaneous Visceral | Cervical Supraclavicular Axilar | Inguinal Perivascular Other locations? |
| Function | Energy storage | Heat production | Adaptative thermogenesis |
| Uncoupling Protein | Nearly undetectable | +++ | ++ (upon stimulation) |
| Vascularization | Low | High | High (upon stimulation) |
| Lipid Content | Tryglicerides | Tryglicerides phopholipids | Tryglicerides phospholipids |
| Impact on Obesity | Positive | Negative | Negative |
3. ‘Beige’ Adipose Tissue
4. Prostate Cancer: A Key Role for Adipose Tissue
5. Adipose Tissue-Tumor Crosstalk. A Role for BAT in Tumorigenesis?
5.1. Leptin
5.2. Adiponectin
6. Adipocytes Modulate Extracellular Matrix Reorganization
6.1. Interleukin-1 (IL-1)
6.2. Interleukin-6 (IL-6)
6.3. Tumor Necrosis Factor Alpha (TNF-α)
6.4. Vascular Endothelial Growth Factor (VEGF)
6.5. Mitochondrial Uncoupling Protein 1(UCP-1)
7. Adipose Tissue and the Pre-Metastatic Niche
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of Adipose Tissue: An Endocrine Organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagergren, K.; Lindam, A.; Lagergren, J. Dietary Proportions of Carbohydrates, Fat, and Protein and Risk of Oesophageal Cancer by Histological Type. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Stenesen, D.; Zeve, D.; Graff, J.M. The Developmental Origins of Adipose Tissue. Development 2013, 140, 3939–3949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimoto, T.; Parton, R.G. Not Just Fat: The Structure and Function of the Lipid Droplet. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.B.; Spiegelman, B.M. ADD1/SREBP1 Promotes Adipocyte Differentiation and Gene Expression Linked to Fatty Acid Metabolism. Genes Dev. 1996, 10, 1096–1107. [Google Scholar] [CrossRef] [Green Version]
- Siersbæk, R.; Nielsen, R.; Mandrup, S. PPARγ in Adipocyte Differentiation and Metabolism—Novel Insights from Genome-Wide Studies. FEBS Lett. 2010, 584, 3242–3249. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Yoshida, N.; Kishimoto, T.; Akira, S. Defective Adipocyte Differentiation in Mice Lacking the C/EBPβ and/or C/EBPδ Gene. EMBO J. 1997, 16, 7432–7443. [Google Scholar] [CrossRef] [Green Version]
- Leitner, B.P.; Huang, S.; Brychta, R.J.; Duckworth, C.J.; Baskin, A.S.; McGehee, S.; Tal, I.; Dieckmann, W.; Gupta, G.; Kolodny, G.M.; et al. Mapping of Human Brown Adipose Tissue in Lean and Obese Young Men. Proc. Natl. Acad. Sci. USA 2017, 114, 8649–8654. [Google Scholar] [CrossRef] [Green Version]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of Fatty-Acid-Dependent UCP1 Uncoupling in Brown Fat Mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, D.G.; Locke, R.M. Thermogenic Mechanisms in Brown Fat. Physiol. Rev. 1984, 64, 1–64. [Google Scholar] [CrossRef] [Green Version]
- Crichton, P.G.; Lee, Y.; Kunji, E.R.S. The Molecular Features of Uncoupling Protein 1 Support a Conventional Mitochondrial Carrier-like Mechanism. Biochimie 2017, 134, 35–50. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Barroso, M.D.M.; Pecqueur, C.; Gelly, C.; Sanchis, D.; Alves-Guerra, M.C.; Bouillaud, F.; Ricquier, D.; Cassard-Doulcier, A.M. Transcriptional Activation of the Human Ucp1 Gene in a Rodent Cell Line. Synergism of Retinoids, Isoproterenol, and Thiazolidinedione Is Mediated by a Multipartite Response Element. J. Biol. Chem. 2000, 275, 31722–31732. [Google Scholar] [CrossRef] [Green Version]
- Teruel, T.; Hernandez, R.; Benito, M.; Lorenzo, M. Rosiglitazone and Retinoic Acid Induce Uncoupling Protein-1 (UCP-1) in a P38 Mitogen-Activated Protein Kinase-Dependent Manner in Fetal Primary Brown Adipocytes. J. Biol. Chem. 2003, 278, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Ong, F.J.; Ahmed, B.A.; Oreskovich, S.M.; Blondin, D.P.; Haq, T.; Konyer, N.B.; Noseworthy, M.D.; Haman, F.; Carpentier, A.C.; Morrison, K.M.; et al. Recent Advances in the Detection of Brown Adipose Tissue in Adult Humans: A Review. Clin. Sci. 2018, 132, 1039–1054. [Google Scholar] [CrossRef]
- Cedikova, M.; Kripnerová, M.; Dvorakova, J.; Pitule, P.; Grundmanova, M.; Babuska, V.; Mullerova, D.; Kuncova, J. Mitochondria in White, Brown, and Beige Adipocytes. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Young, P.; Arch, J.R.S.; Ashwell, M. Brown Adipose Tissue in the Parametrial Fat Pad of the Mouse. FEBS Lett. 1984, 167, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Nedergaard, J.; Cannon, B. The Browning of White Adipose Tissue: Some Burning Issues. Cell Metab. 2014, 20, 396–407. [Google Scholar] [CrossRef] [Green Version]
- Chawta, A.; Repa, J.J.; Evans, R.M.; Mangelsdorf, D.J. Nuclear Receptors and Lipid Physiology: Opening the x-Files. Science 2001, 294, 1866–1870. [Google Scholar]
- David, L.; Feige, J.J.; Bailly, S. Emerging Role of Bone Morphogenetic Proteins in Angiogenesis. Cytokine Growth Factor Rev. 2009, 20, 203–212. [Google Scholar] [CrossRef]
- Lim, S.; Honek, J.; Xue, Y.; Seki, T.; Cao, Z.; Andersson, P.; Yang, X.; Hosaka, K.; Cao, Y. Cold-Induced Activation of Brown Adipose Tissue and Adipose Angiogenesis in Mice. Nat. Protoc. 2012, 7, 606–615. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, C.; Wang, H.; Foss, R.M.; Clare, M.; George, E.V.; Li, S.; Katz, A.; Cheng, H.; Ding, Y.; et al. Irisin Exerts Dual Effects on Browning and Adipogenesis of Human White Adipocytes. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E530–E541. [Google Scholar] [CrossRef]
- Kuryłowicz, A.; Puzianowska-Kuźnicka, M. Induction of Adipose Tissue Browning as a Strategy to Combat Obesity. Int. J. Mol. Sci. 2020, 21, 6241. [Google Scholar] [CrossRef]
- Collins, S. β-Adrenoceptor Signaling Networks in Adipocytes for Recruiting Stored Fat and Energy Expenditure. Front. Endocrinol. 2012, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, O.; Noda, T.; Morita, A.; Morita, M.; Ohtsuki, H.; Sugiyama, M.; Funaba, M. Castration Induced Browning in Subcutaneous White Adipose Tissue in Male Mice. Biochem. Biophys. Res. Commun. 2016, 478, 1746–1750. [Google Scholar] [CrossRef]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Rajan, S.; Gupta, A.; Beg, M.; Shankar, K.; Srivastava, A.; Varshney, S.; Kumar, D.; Gaikwad, A.N. Adipocyte Transdifferentiation and Its Molecular Targets. Differentiation 2014, 87, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. Reversible Physiological Transdifferentiation in the Adipose Organ. Proc. Nutr. Soc. 2009, 68, 340–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontini, A.; Vitali, A.; Perugini, J.; Murano, I.; Romiti, C.; Guerrieri, M.; Cinti, S. WAT to BAT Transdifferentiation of Omental Fat in Adult Humans Affected by Pheochromocytomas. Ital. J. Anat. Embryol. 2013, 118, 94. [Google Scholar]
- Daas, S.I.; Rizeq, B.R.; Nasrallah, G.K. Adipose Tissue Dysfunction in Cancer Cachexia. J. Cell. Physiol. 2018, 234, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Abbott, D.E.; Pritchard, C.; Clegg, N.J.; Ferguson, C.; Dumpit, R.; Sikes, R.A.; Nelson, P.S. Expressed Sequence Tag Profiling Identifies Developmental and Anatomic Partitioning of Gene Expression in the Mouse Prostate. Genome Biol. 2003, 4, R79. [Google Scholar] [CrossRef] [Green Version]
- Allott, E.H.; Masko, E.M.; Freedland, S.J. Obesity and Prostate Cancer: Weighing the Evidence. Eur. Urol. 2013, 63, 800–809. [Google Scholar] [CrossRef] [Green Version]
- Mehlem, A.; Hagberg, C.E.; Muhl, L.; Eriksson, U.; Falkevall, A. Imaging of Neutral Lipids by Oil Red O for Analyzing the Metabolic Status in Health and Disease. Nat. Protoc. 2013, 8, 1149–1154. [Google Scholar] [CrossRef] [Green Version]
- Bunney, P.E.; Zink, A.N.; Holm, A.A.; Billington, C.J.; Kotz, C.M. Orexin Activation Counteracts Decreases in Nonexercise Activity Thermogenesis (NEAT) Caused by High-Fat Diet. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Park, J.; Euhus, D.M.; Scherer, P.E. Paracrine and Endocrine Effects of Adipose Tissue on Cancer Development and Progression. Endocr. Rev. 2011, 32, 550–570. [Google Scholar] [CrossRef] [Green Version]
- Hurwitz, A.A.; Foster, B.A.; Allison, J.P.; Greenberg, N.M.; Kwon, E.D. The TRAMP mouse as a model for prostate cancer. In Current Protocols in Immunology; John, E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; Chapter 20, Unit 20.5. [Google Scholar]
- Wu, Q.; Li, B.; Li, Z.; Li, J.; Sun, S.; Sun, S. Cancer-Associated Adipocytes: Key Players in Breast Cancer Progression. J. Hematol. Oncol. 2019, 12, 1–15. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Artis, D. Immune Regulation of Metabolic Homeostasis in Health and Disease. Cell 2015, 161, 146–160. [Google Scholar] [CrossRef] [Green Version]
- Howe, L.R.; Subbaramaiah, K.; Hudis, C.A.; Dannenberg, A.J. Molecular Pathways: Adipose Inflammation as a Mediator of Obesity-Associated Cancer. Clin. Cancer Res. 2013, 19, 6074–6083. [Google Scholar] [CrossRef] [Green Version]
- Shankar, K.; Kumar, D.; Gupta, S.; Varshney, S.; Rajan, S.; Srivastava, A.; Gupta, A.; Gupta, A.P.; Vishwakarma, A.L.; Gayen, J.R.; et al. Role of Brown Adipose Tissue in Modulating Adipose Tissue Inflammation and Insulin Resistance in High-Fat Diet Fed Mice. Eur. J. Pharmacol. 2019, 854, 354–364. [Google Scholar] [CrossRef]
- Stanford, K.I.; Middelbeek, R.J.W.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.H.; et al. Brown Adipose Tissue Regulates Glucose Homeostasis and Insulin Sensitivity. J. Clin. Invest. 2013, 123, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.D.; Qiu, Y.; Cui, X.; Goh, Y.P.S.; Mwangi, J.; David, T.; Mukundan, L.; Brombacher, F.; Locksley, R.M.; Chawla, A. Alternatively Activated Macrophages Produce Catecholamines to Sustain Adaptive Thermogenesis. Nature 2011, 480, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Sierra, H.; Cordova, M.; Chen, C.-S.J.; Rajadhyaksha, M. Confocal Imaging–Guided Laser Ablation of Basal Cell Carcinomas: An Ex Vivo Study. J. Invest. Dermatol. 2015, 135, 612–615. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.; Koch, M.O.; Foster, R.S.; Bihrle, R.; Gardner, T.A.; Fyffe, J.; Ulbright, T.M.; Eble, J.N.; Cheng, L. Anatomic Distribution of Periprostatic Adipose Tissue: A Mapping Study of 100 Radical Prostatectomy Specimens. Cancer 2003, 97, 1639–1643. [Google Scholar] [CrossRef]
- Uehara, H.; Kobayashi, T.; Matsumoto, M.; Watanabe, S.; Yoneda, A.; Bando, Y. Adipose Tissue: Critical Contributor to the Development of Prostate Cancer. J. Med. Investig. 2018, 65, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Su, F.; Ahn, S.; Saha, A.; DiGiovanni, J.; Kolonin, M.G. Adipose Stromal Cell Targeting Suppresses Prostate Cancer Epithelial-Mesenchymal Transition and Chemoresistance. Oncogene 2019, 38, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Estève, D.; Roumiguié, M.; Manceau, C.; Milhas, D.; Muller, C. Periprostatic Adipose Tissue: A Heavy Player in Prostate Cancer Progression. Curr. Opin. Endocr. Metab. Res. 2020, 10, 29–35. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Xie, C.; Chen, Z.; Liu, Y.; Ru, F.; He, Y. Leptin Promotes Epithelial-Mesenchymal Transition in Benign Prostatic Hyperplasia through Downregulation of BAMBI. Exp. Cell Res. 2020, 387, 111754. [Google Scholar] [CrossRef]
- Fu, S.; Xu, H.; Gu, M.; Liu, C.; Wang, Q.; Wan, X.; Chen, Y.; Chen, Q.; Peng, Y.; Cai, Z.; et al. Adiponectin Deficiency Contributes to the Development and Progression of Benign Prostatic Hyperplasia in Obesity. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Sacca, P.A.; Mazza, O.N.; Scorticati, C.; Vitagliano, G.; Casas, G.; Calvo, J.C. Human Periprostatic Adipose Tissue: Secretome from Patients with Prostate Cancer or Benign Prostate Hyperplasia. Cancer Genom. Proteom. 2019, 16, 29–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triantafyllou, G.A.; Paschou, S.A.; Mantzoros, C.S. Leptin and Hormones: Energy Homeostasis. Endocrinol. Metab. Clin. North Am. 2016, 45, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Paulus, K.; Jöhren, O.; Lehnert, H. Intranasal Leptin Reduces Appetite and Induces Weight Loss in Rats with Diet-Induced Obesity (DIO). Endocrinology 2012, 153, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.; Beerens, S.; Adan, R.A.H. Role of Leptin in Energy Expenditure: The Hypothalamic Perspective. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R938–R947. [Google Scholar] [CrossRef] [Green Version]
- Dieudonne, M.N.; Machinal-Quelin, F.; Serazin-Leroy, V.; Leneveu, M.C.; Pecquery, R.; Giudicelli, Y. Leptin Mediates a Proliferative Response in Human MCF7 Breast Cancer Cells. Biochem. Biophys. Res. Commun. 2002, 293, 622–628. [Google Scholar] [CrossRef]
- Gonzalez, R.R.; Cherfils, S.; Escobar, M.; Yoo, J.H.; Carino, C.; Styer, A.K.; Sullivan, B.T.; Sakamoto, H.; Olawaiye, A.; Serikawa, T.; et al. Leptin Signaling Promotes the Growth of Mammary Tumors and Increases the Expression of Vascular Endothelial Growth Factor (VEGF) and Its Receptor Type Two (VEGF-R2). J. Biol. Chem. 2006, 281, 26320–26328. [Google Scholar] [CrossRef] [Green Version]
- Aparicio, T.; Kotelevets, L.; Tsocas, A.; Laigneau, J.P.; Sobhani, I.; Chastre, E.; Lehy, T. Leptin Stimulates the Proliferation of Human Colon Cancer Cells in Vitro but Does Not Promote the Growth of Colon Cancer Xenografts in Nude Mice or Intestinal Tumorigenesis in ApcMin/+ Mice. Gut 2005, 54, 1136–1145. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Wei, L.; Huang, Y.; Wu, Y.; Su, M.; Pang, X.; Wang, N.; Ji, F.; Zhong, C.; Chen, T. Leptin Promotes Breast Cancer Cell Migration and Invasion via IL-18 Expression and Secretion. Int. J. Oncol. 2016, 48, 2479–2487. [Google Scholar] [CrossRef] [Green Version]
- Hoda, M.R.; Theil, G.; Mohammed, N.; Fischer, K.; Fornara, P. The Adipocyte-Derived Hormone Leptin Has Proliferative Actions on Androgen-Resistant Prostate Cancer Cells Linking Obesity to Advanced Stages of Prostate Cancer. J. Oncol. 2012. [Google Scholar] [CrossRef]
- Stattin, P.; Söderberg, S.; Hallmans, G.; Bylund, A.; Kaaks, R.; Stenman, U.H.; Bergh, A.; Olsson, T. Leptin Is Associated with Increased Prostate Cancer Risk: A Nested Case-Referent Study. J. Clin. Endocrinol. Metab. 2001, 86, 1341–1345. [Google Scholar] [CrossRef]
- Gupta, A.; Herman, Y.; Ayers, C.; Beg, M.S.; Lakoski, S.G.; Abdullah, S.M.; Johnson, D.H.; Neeland, I.J. Plasma Leptin Levels and Risk of Incident Cancer: Results from the Dallas Heart Study. PLoS ONE 2016, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [Green Version]
- Parker-Duffen, J.L.; Nakamura, K.; Silver, M.; Zuriaga, M.A.; Maclauchlan, S.; Aprahamian, T.R.; Walsh, K. Divergent Roles for Adiponectin Receptor 1 (Adipor1) and AdipoR2 in Mediating Revascularization and Metabolic Dysfunction in Vivo. J. Biol. Chem. 2014, 289, 16200–16213. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, Q.; Pu, H.; Wei, Q.; Duan, M.; Zhang, C.; Jiang, T.; Shou, X.; Zhang, J.; Yang, Y. Adiponectin Improves NF-ΚB-Mediated Inflammation and Abates Atherosclerosis Progression in Apolipoprotein E-Deficient Mice. Lipids Health Dis. 2016, 15, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Zheng, J.; Yao, X.; Peng, B. Adiponectin Inhibits VEGF-A in Prostate Cancer Cells. Tumor Biol. 2015, 36, 4287–4292. [Google Scholar] [CrossRef]
- Katira, A.; Tan, P.H. Evolving Role of Adiponectin in Cancer-Controversies and Update. Cancer Biol. Med. 2016, 13, 101–119. [Google Scholar] [CrossRef] [Green Version]
- Richard, A.J.; Stephens, J.M. The Role of JAK–STAT Signaling in Adipose Tissue Function. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, T.; Endo, H.; Tomimoto, A.; Sugiyama, M.; Takahashi, H.; Saito, S.; Inamori, M.; Nakajima, N.; Watanabe, M.; Kubota, N.; et al. Adiponectin Suppresses Colorectal Carcinogenesis under the High-Fat Diet Condition. Gut 2008, 57, 1531–1538. [Google Scholar] [CrossRef] [Green Version]
- Zangani, D.; Darcy, K.M.; Shoemaker, S.; Ip, M.M. Adipocyte-Epithelial Interactions Regulate the in Vitro Development of Normal Mammary Epithelial Cells. Exp. Cell Res. 1999, 247, 399–409. [Google Scholar] [CrossRef]
- Hu, X.; Juneja, S.C.; Maihle, N.J.; Cleary, M.P. Leptin—A Growth Factor in Normal and Malignant Breast Cells and for Normal Mammary Gland Development. J. Natl. Cancer Inst. 2002, 94, 1704–1711. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Van De Wall, E.; Laplante, M.; Azzara, A.; Trujillo, M.E.; Hofmann, S.M.; Schraw, T.; Durand, J.L.; Li, H.; Li, G.; et al. Obesity-Associated Improvements in Metabolic Profile through Expansion of Adipose Tissue. J. Clin. Invest. 2007, 117, 2621–2637. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, A.R.; Coffer, P.J. A Tumor Suppressor Role for C/EBPα in Solid Tumors: More than Fat and Blood. Oncogene 2017, 36, 5221–5230. [Google Scholar] [CrossRef] [PubMed]
- Saez, E.; Rosenfeld, J.; Livolsi, A.; Olson, P.; Lombardo, E.; Nelson, M.; Banayo, E.; Cardiff, R.D.; Izpisua-Belmonte, J.C.; Evans, R.M. PPARγ Signaling Exacerbates Mammary Gland Tumor Development. Genes Dev. 2004, 18, 528–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirillo, D.; Rachiglio, A.M.; La Montagna, R.; Giordano, A.; Normanno, N. Leptin Signaling in Breast Cancer: An Overview. J. Cell. Biochem. 2008, 105, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Soma, D.; Kitayama, J.; Yamashita, H.; Miyato, H.; Ishikawa, M.; Nagawa, H. Leptin Augments Proliferation of Breast Cancer Cells via Transactivation of HER2. J. Surg. Res. 2008, 149, 9–14. [Google Scholar] [CrossRef]
- Kothari, C.; Diorio, C.; Durocher, F. The Importance of Breast Adipose Tissue in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 5760. [Google Scholar] [CrossRef]
- Ortega-Molina, A.; Serrano, M. PTEN in Cancer, Metabolism, and Aging. Trends Endocrinol. Metab. 2013, 24, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.X.; Cao, L.Y.; Chen, X.; Xiao, J.; Zou, Y.; Chen, Q. PTEN Inhibits Cell Proliferation, Promotes Cell Apoptosis, and Induces Cell Cycle Arrest via Downregulating the PI3K/AKT/ HTERT Pathway in Lung Adenocarcinoma A549 Cells. Biomed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Vaitkus, J.A.; Celi, F.S. The Role of Adipose Tissue in Cancer-Associated Cachexia. Exp. Biol. Med. 2017, 242, 473–481. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Méndez-Gutiérrez, A.; Aguilera, C.M.; Plaza-Díaz, J. Extracellular Matrix Remodeling of Adipose Tissue in Obesity and Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 4888. [Google Scholar] [CrossRef] [Green Version]
- Schoettl, T.; Fischer, I.P.; Ussar, S. Heterogeneity of Adipose Tissue in Development and Metabolic Function. J. Exp. Biol. 2018, 121, jeb162958. [Google Scholar] [CrossRef] [Green Version]
- Walker, C.; Mojares, E.; Del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 103028. [Google Scholar] [CrossRef] [Green Version]
- Occhino, F.; Stehulak, T. Behind the Slow Pace of Wage Growth. Economic Trends 2015, 115, 8–10. [Google Scholar]
- Liu, R.; Nikolajczyk, B.S. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front. Immunol. 2019, 10, 1587. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. Overview of the IL-1 Family in Innate Inflammation and Acquired Immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Croston, G.E.; Cao, Z.; Goeddel, D.V. NF-ΚB Activation by Interleukin-1 (IL-1) Requires an IL-1 Receptor- Associated Protein Kinase Activity. J. Biol. Chem. 1995, 270, 16514–16517. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.; Gerdes, N.; Fritzenwanger, M.; Figulla, H.R. Circulating Levels of Interleukin-1 Family Cytokines in Overweight Adolescents. Mediat. Inflamm. 2010, 2010, 1–6. [Google Scholar] [CrossRef]
- Shoda, H.; Nagafuchi, Y.; Tsuchida, Y.; Sakurai, K.; Sumitomo, S.; Fujio, K.; Yamamoto, K. Increased Serum Concentrations of IL-1 Beta, IL-21 and Th17 Cells in Overweight Patients with Rheumatoid Arthritis. Arthritis Res. Ther. 2017, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Keku, T.O.; Martin, C.; Galanko, J.; Woosley, J.T.; Schroeder, J.C.; Satia, J.A.; Halabi, S.; Sandler, R.S. Circulating Levels of Inflammatory Cytokines and Risk of Colorectal Adenomas. Cancer Res. 2008, 68, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Il’yasova, D.; Colbert, L.H.; Harris, T.B.; Newman, A.B.; Bauer, D.C.; Satterfield, S.; Kritchevsky, S.B. Circulating Levels of Inflammatory Markers and Cancer Risk in the Health Aging and Body Composition Cohort. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 2413–2418. [Google Scholar] [CrossRef] [Green Version]
- Juge-Aubry, C.E.; Somm, E.; Giusti, V.; Pernin, A.; Chicheportiche, R.; Verdumo, C.; Rohner-Jeanrenaud, F.; Burger, D.; Dayer, J.M.; Meier, C.A. Adipose Tissue Is a Major Source of Interleukin-1 Receptor Antagonist: Upregulation in Obesity and Inflammation. Diabetes 2003, 52, 1104–1110. [Google Scholar] [CrossRef] [Green Version]
- Dudás, J.; Fullár, A.; Bitsche, M.; Schartinger, V.; Kovalszky, I.; Sprinzl, G.M.; Riechelmann, H. Tumor-Produced, Active Interleukin-1 β Regulates Gene Expression in Carcinoma-Associated Fibroblasts. Exp. Cell Res. 2011, 317, 2222–2229. [Google Scholar] [CrossRef] [Green Version]
- Lewis, A.M.; Varghese, S.; Xu, H.; Alexander, H.R. Interleukin-1 and Cancer Progression: The Emerging Role of Interleukin-1 Receptor Antagonist as a Novel Therapeutic Agent in Cancer Treatment. J. Transl. Med. 2006, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Russell, M.R.; Shahriari, K.; Jernigan, D.L.; Lioni, M.I.; Garcia, F.U.; Fatatis, A. Interleukin-1β Promotes Skeletal Colonization and Progression of Metastatic Prostate Cancer Cells with Neuroendocrine Features. Cancer Res. 2013, 73, 3297–3305. [Google Scholar] [CrossRef] [Green Version]
- Diaz, M.; Abdul, M.; Hoosein, N. Modulation of Neuroendocrine Differentiation in Prostate Cancer by Interleukin-1 and -2. Prostate 1998, 36, 32–36. [Google Scholar] [CrossRef]
- Sainz, R.M.; Mayo, J.C.; Tan, D.X.; León, J.; Manchester, L.; Reiter, R.J. Melatonin Reduces Prostate Cancer Cell Growth Leading to Neuroendocrine Differentiation via a Receptor and PKA Independent Mechanism. Prostate 2005, 63, 29–43. [Google Scholar] [CrossRef]
- Timper, K.; Denson, J.L.; Steculorum, S.M.; Heilinger, C.; Engström-Ruud, L.; Wunderlich, C.M.; Rose-John, S.; Wunderlich, F.T.; Brüning, J.C. IL-6 Improves Energy and Glucose Homeostasis in Obesity via Enhanced Central IL-6 Trans-Signaling. Cell Rep. 2017, 19, 267–280. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.P.; Li, J.; Tewari, A.K. Inflammation and Prostate Cancer: The Role of Interleukin 6 (IL-6). BJU Int. 2014, 113, 986–992. [Google Scholar] [CrossRef]
- Okamoto, M.; Lee, C.; Oyasu, R. Interleukin-6 as a Paracrine and Autocrine Growth Factor in Human Prostatic Carcinoma Cells in Vitro. Cancer Res. 1997, 57, 141–146. [Google Scholar]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF Biology, Pathogenic Mechanisms and Emerging Therapeutic Strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Sedger, L.M.; McDermott, M.F. TNF and TNF-Receptors: From Mediators of Cell Death and Inflammation to Therapeutic Giants—Past, Present and Future. Cytokine Growth Factor Rev. 2014, 25, 453–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory Cytokines IL-6 and TNF-α and the Development of Inflammation in Obese Subjects. Eur. J. Med. Res. 2010, 15, 120. [Google Scholar] [CrossRef]
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S.W. Subcutaneous Adipose Tissue Releases Interleukin-6, but Not Tumor Necrosis Factor-α, in Vivo. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200. [Google Scholar] [CrossRef]
- Tse, B.W.C.; Scott, K.F.; Russell, P.J. Paradoxical Roles of Tumour Necrosis Factor-Alpha in Prostate Cancer Biology. Prostate Cancer 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Maolake, A.; Izumi, K.; Natsagdorj, A.; Iwamoto, H.; Kadomoto, S.; Makino, T.; Naito, R.; Shigehara, K.; Kadono, Y.; Hiratsuka, K.; et al. Tumor Necrosis Factor-α Induces Prostate Cancer Cell Migration in Lymphatic Metastasis through CCR7 Upregulation. Cancer Sci. 2018, 109, 1524–1531. [Google Scholar] [CrossRef]
- Chopra, D.P.; Menard, R.E.; Januszewski, J.; Mattingly, R.R. TNF-α-Mediated Apoptosis in Normal Human Prostate Epithelial Cells and Tumor Cell Lines. Cancer Lett. 2004, 203, 145–154. [Google Scholar] [CrossRef]
- Villarroya, F.; Gavaldà-Navarro, A.; Peyrou, M.; Villarroya, J.; Giralt, M. The Lives and Times of Brown Adipokines. Trends Endocrinol. Metab. 2017, 28, 855–867. [Google Scholar] [CrossRef]
- Wang, G.X.; Zhao, X.Y.; Lin, J.D. The Brown Fat Secretome: Metabolic Functions beyond Thermogenesis. Trends Endocrinol. Metab. 2015, 26, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Gunawardana, S.C. Therapeutic Value of Brown Adipose Tissue. Adipocyte 2012, 1, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.X.; Zhao, X.Y.; Meng, Z.X.; Kern, M.; Dietrich, A.; Chen, Z.; Cozacov, Z.; Zhou, D.; Okunade, A.L.; Su, X.; et al. The Brown Fat-Enriched Secreted Factor Nrg4 Preserves Metabolic Homeostasis through Attenuation of Hepatic Lipogenesis. Nat. Med. 2014, 20, 1436–1443. [Google Scholar] [CrossRef]
- Hondares, E.; Iglesias, R.; Giralt, A.; Gonzalez, F.J.; Giralt, M.; Mampel, T.; Villarroya, F. Thermogenic Activation Induces FGF21 Expression and Release in Brown Adipose Tissue. J. Biol. Chem. 2011, 286, 12983–12990. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Kusminski, C.M.; Luby-Phelps, K.; Spurgin, S.B.; An, Y.A.; Wang, Q.A.; Holland, W.L.; Scherer, P.E. Brown Adipose Tissue Derived VEGF-A Modulates Cold Tolerance and Energy Expenditure. Mol. Metab. 2014, 3, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New Role of Bone Morphogenetic Protein 7 in Brown Adipogenesis and Energy Expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef]
- Stefanini, M.O.; Wu, F.T.H.; Mac Gabhann, F.; Popel, A.S. A Compartment Model of VEGF Distribution in Blood, Healthy and Diseased Tissues. BMC Syst. Biol. 2008, 2, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, I.; Franckhauser, S.; Ferré, T.; Vilà, L.; Tafuro, S.; Muñoz, S.; Roca, C.; Ramos, D.; Pujol, A.; Riu, E.; et al. Adipose Tissue Overexpression of Vascular Endothelial Growth Factor Protects against Diet-Induced Obesity and Insulin Resistance. Diabetes 2012, 61, 1801–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aase, K.; Lymboussaki, A.; Kaipainen, A.; Olofsson, B.; Alitalo, K.; Eriksson, U. Localization of VEGF-B in the Mouse Embryo Suggests a Paracrine Role of the Growth Factor in the Developing Vasculature. Dev. Dyn. 1999, 215, 12–25. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Sun, K.; An, Y.A.; Gu, X.; Scherer, P.E. VEGF-A—Expressing Adipose Tissue Shows Rapid Beiging and Enhanced Survival after Transplantation and Confers IL-4-Independent Metabolic Improvements. Diabetes 2017, 66, 1479–1490. [Google Scholar] [CrossRef] [Green Version]
- Chekhonin, V.P.; Shein, S.A.; Korchagina, A.A.; Gurina, O.I. VEGF in Tumor Progression and Targeted Therapy. Curr. Cancer Drug Targets 2013, 13, 423–443. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef]
- Carreira, A.C.; Alves, G.G.; Zambuzzi, W.F.; Sogayar, M.C.; Granjeiro, J.M. Bone Morphogenetic Proteins: Structure, Biological Function and Therapeutic Applications. Arch. Biochem. Biophys. 2014, 561, 64–73. [Google Scholar] [CrossRef]
- Schulz, T.J.; Tseng, Y.H. Emerging Role of Bone Morphogenetic Proteins in Adipogenesis and Energy Metabolism. Cytokine Growth Factor Rev. 2009, 20, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, A.; Okuda, H.; Xing, F.; Pandey, P.R.; Watabe, M.; Hirota, S.; Pai, S.K.; Liu, W.; Fukuda, K.; Chambers, C.; et al. Bone Morphogenetic Protein 7 in Dormancy and Metastasis of Prostate Cancer Stem-like Cells in Bone. J. Exp. Med. 2011, 208, 2641–2655. [Google Scholar] [CrossRef] [Green Version]
- Buijs, J.T.; Rentsch, C.A.; Van Der Horst, G.; Van Overveld, P.G.M.; Wetterwald, A.; Schwaninger, R.; Henriquez, N.V.; Ten Dijke, P.; Borovecki, F.; Markwalder, R.; et al. BMP7, a Putative Regulator of Epithelial Homeostasis in the Human Prostate, Is a Potent Inhibitor of Prostate Cancer Bone Metastasis in Vivo. Am. J. Pathol. 2007, 171, 1047–1057. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.; Anderson, W.A.; Raman, V.; Reddi, A.H. Androgen-Dependent Gene Expression of Bone Morphogenetic Protein 7 in Mouse Prostate. Prostate 1998, 37, 236–245. [Google Scholar] [CrossRef]
- Fournier, P.G.J.; Guise, T.A. BMP7: A New Bone Metastases Prevention? Am. J. Pathol. 2007, 171, 739–743. [Google Scholar] [CrossRef] [Green Version]
- Mailloux, R.J.; Harper, M.E. Uncoupling Proteins and the Control of Mitochondrial Reactive Oxygen Species Production. Free Radic. Biol. Med. 2011, 51, 1106–1115. [Google Scholar] [CrossRef]
- Ali Khan, A.; Hansson, J.; Weber, P.; Foehr, S.; Krijgsveld, J.; Herzig, S.; Scheideler, M. Comparative Secretome Analyses of Primary Murine White and Brown Adipocytes Reveal Novel Adipokines. Mol. Cell. Proteom. 2018, 17, 2358–2370. [Google Scholar] [CrossRef] [Green Version]
- Valle, I.; Álvarez-Barrientos, A.; Arza, E.; Lamas, S.; Monsalve, M. PGC-1α Regulates the Mitochondrial Antioxidant Defense System in Vascular Endothelial Cells. Cardiovasc. Res. 2005, 66, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Oelkrug, R.; Goetze, N.; Meyer, C.W.; Jastroch, M. Antioxidant Properties of UCP1 Are Evolutionarily Conserved in Mammals and Buffer Mitochondrial Reactive Oxygen Species. Free Radic. Biol. Med. 2014, 77, 210–216. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Plerre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide Activates Mitochondrial Uncoupling Proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef]
- Hondares, E.; Rosell, M.; Díaz-Delfín, J.; Olmos, Y.; Monsalve, M.; Iglesias, R.; Villarroya, F.; Giralt, M. Peroxisome Proliferator-Activated Receptor α (PPARα) Induces PPARγ Coactivator 1α (PGC-1α) Gene Expression and Contributes to Thermogenic Activation of Brown Fat: Involvement of PRDM16. J. Biol. Chem. 2011, 286, 43112–43122. [Google Scholar] [CrossRef] [Green Version]
- Koen, E.J.; Collier, A.B. Particle-in-Cell Simulations of a Beam Driven Plasma. Phys. Plasmas 2010, 19, 1420–1428. [Google Scholar]
- Micalizzi, D.S.; Ford, H.L. Epithelial-Mesenchymal Transition in Development and Cancer. Future Oncol. 2009, 5, 1129–1143. [Google Scholar] [CrossRef]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [Green Version]
- Tsubakihara, Y.; Moustakas, A. Epithelial-Mesenchymal Transition and Metastasis under the Control of Transforming Growth Factor β. Int. J. Mol. Sci. 2018, 19, 3672. [Google Scholar] [CrossRef] [Green Version]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The Role of Adiponectin in Cancer: A Review of Current Evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, Y.; Funahashi, T.; Kihara, S.; Taguchi, T.; Tamaki, Y.; Matsuzawa, Y.; Noguchi, S. Association of Serum Adiponectin Levels with Breast Cancer Risk. Clin. Cancer Res. 2003, 9, 5699–5704. [Google Scholar]
- Macis, D.; Gandini, S.; Guerrieri-Gonzaga, A.; Johansson, H.; Magni, P.; Ruscica, M.; Lazzeroni, M.; Serrano, D.; Cazzaniga, M.; Mora, S.; et al. Prognostic Effect of Circulating Adiponectin in a Randomized 2 × 2 Trial of Low-Dose Tamoxifen and Fenretinide in Premenopausal Women at Risk for Breast Cancer. J. Clin. Oncol. 2012, 30, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.C.; Chang, Y.T.; Su, T.C.; Yang, W.S.; Chen, C.L.; Tien, Y.W.; Liang, P.C.; Wei, S.C.; Wong, J.M. Adiponectin as a Potential Differential Marker to Distinguish Pancreatic Cancer and Chronic Pancreatitis. Pancreas 2007, 35, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Stolzenberg-Solomon, R.Z.; Weinstein, S.; Pollak, M.; Tao, Y.; Taylor, P.R.; Virtamo, J.; Albanes, D. Prediagnostic Adiponectin Concentrations and Pancreatic Cancer Risk in Male Smokers. Am. J. Epidemiol. 2008, 168, 1047–1055. [Google Scholar] [CrossRef]
- Arano, T.; Nakagawa, H.; Tateishi, R.; Ikeda, H.; Uchino, K.; Enooku, K.; Goto, E.; Masuzaki, R.; Asaoka, Y.; Kondo, Y.; et al. Serum Level of Adiponectin and the Risk of Liver Cancer Development in Chronic Hepatitis C Patients. Int. J. Cancer 2011, 129, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Michalakis, K.; Williams, C.J.; Mitsiades, N.; Blakeman, J.; Balafouta-Tselenis, S.; Giannopoulos, A.; Mantzoros, C.S. Serum Adiponectin Concentrations and Tissue Expression of Adiponectin Receptors Are Reduced in Patients with Prostate Cancer: A Case Control Study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 308–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Stampfer, M.J.; Mucci, L.; Rifai, N.; Qiu, W.; Kurth, T.; Ma, J. A 25-Year Prospective Study of Plasma Adiponectin and Leptin Concentrations and Prostate Cancer Risk and Survival. Clin. Chem. 2010, 56, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petridou, E.T.; Mitsiades, N.; Gialamas, S.; Angelopoulos, M.; Skalkidou, A.; Dessypris, N.; Hsi, A.; Lazaris, N.; Polyzos, A.; Syrigos, C.; et al. Circulating Adiponectin Levels and Expression of Adiponectin Receptors in Relation to Lung Cancer: Two Case-Control Studies. Oncology 2008, 73, 261–269. [Google Scholar] [CrossRef]
- Mantzoros, C.S.; Trakatelli, M.; Gogas, H.; Dessypris, N.; Stratigos, A.; Chrousos, G.P.; Petridou, E.T. Circulating Adiponectin Levels in Relation to Melanoma: A Case-Control Study. Eur. J. Cancer 2007, 43, 1430–1436. [Google Scholar] [CrossRef]
- Park, Y.; Kim, J.W.; Kim, D.S.; Kim, E.B.; Park, S.J.; Park, J.Y.; Choi, W.S.; Song, J.G.; Seo, H.Y.; Oh, S.C.; et al. The Bone Morphogenesis Protein-2 (BMP-2) Is Associated with Progression to Metastatic Disease in Gastric Cancer. Cancer Res. Treat. 2008, 40, 127. [Google Scholar] [CrossRef] [Green Version]
- Riehn, M.; Klopocki, E.; Molkentin, M.; Reinhardt, R.; Burmeister, T. A BACH2-BCL2L1 Fusion Gene Resulting from a Lymphoma Cell Line BLUE-1. Cancer 2011, 396, 389–396. [Google Scholar]
- Buijs, J.T.; Henriquez, N.V.; Van Overveld, P.G.M.; Van Der Horst, G.; Que, I.; Schwaninger, R.; Rentsch, C.; Ten Dijke, P.; Cleton-Jansen, A.M.; Driouch, K.; et al. Bone Morphogenetic Protein 7 in the Development and Treatment of Bone Metastases from Breast Cancer. Cancer Res. 2007, 67, 8742–8751. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, S.; Alarmo, E.L.; Sandström, S.; Ampuja, M.; Kallioniemi, A. Bone Morphogenetic Protein -4 and -5 in Pancreatic Cancer-Novel Bidirectional Players. Exp. Cell Res. 2011, 317, 2136–2146. [Google Scholar] [CrossRef]
- Wu, G.; Huang, F.; Chen, Y.; Zhuang, Y.; Huang, Y.; Xie, Y. High Levels of BMP2 Promote Liver Cancer Growth via the Activation of Myeloid-Derived Suppressor Cells. Front. Oncol. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Španjol, J.; Djordjević, G.; Markić, D.; Klarić, M.; Fučkar, D.; Bobinac, D. Role of Bone Morphogenetic Proteins in Human Prostate Cancer Pathogenesis and Development of Bone Metastases: Immunohistochemical Study. Coll. Antropol. 2010, 34, 119–125. [Google Scholar]
- Bieniasz, M.; Oszajca, K.; Eusebio, M.; Kordiak, J.; Bartkowiak, J.; Szemraj, J. The Positive Correlation between Gene Expression of the Two Angiogenic Factors: VEGF and BMP-2 in Lung Cancer Patients. Lung Cancer 2009, 66, 319–326. [Google Scholar] [CrossRef]
- Chen, J.; Ye, L.; Xie, F.; Yang, Y.; Zhang, L.; Jiang, W.G. Expression of Bone Morphogenetic Protein 7 in Lung Cancer and Its Biological Impact on Lung Cancer Cells. Anticancer Res. 2010, 30, 1113–1120. [Google Scholar]
- Rothhammer, T.; Poser, I.; Soncin, F.; Bataille, F.; Moser, M.; Bosserhoff, A.K. Bone Morphogenic Proteins Are Overexpressed in Malignant Melanoma and Promote Cell Invasion and Migration. Cancer Res. 2005, 65, 448–456. [Google Scholar]
- Lin, K.; Zou, R.; Lin, F.; Zheng, S.; Shen, X.; Xue, X. Expression and Effect of CXCL14 in Colorectal Carcinoma. Mol. Med. Rep. 2014, 10, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.L.; Ou, Z.L.; Lin, F.J.; Yang, X.L.; Luo, J.M.; Shen, Z.Z.; Shao, Z.M. Expression of CXCL14 and Its Anticancer Role in Breast Cancer. Breast Cancer Res. Treat. 2012, 135, 725–735. [Google Scholar] [CrossRef]
- Westrich, J.A.; Vermeer, D.W.; Colbert, P.L.; Spanos, W.C.; Pyeon, D. The Multifarious Roles of the Chemokine CXCL14 in Cancer Progression and Immune Responses. Mol. Carcinog. 2020, 59, 794–806. [Google Scholar] [CrossRef]
- Wente, M.N.; Mayer, C.; Gaida, M.M.; Michalski, C.W.; Giese, T.; Bergmann, F.; Giese, N.A.; Büchler, M.W.; Friess, H. CXCL14 Expression and Potential Function in Pancreatic Cancer. Cancer Lett. 2008, 259, 209–217. [Google Scholar] [CrossRef]
- Wang, W.; Huang, P.; Zhang, L.; Wei, J.; Xie, Q.; Sun, Q.; Zhou, X.; Xie, H.; Zhou, L.; Zheng, S. Antitumor Efficacy of C-X-C Motif Chemokine Ligand 14 in Hepatocellular Carcinoma in Vitro and in Vivo. Cancer Sci. 2013, 104, 1523–1531. [Google Scholar] [CrossRef]
- Augsten, M.; Hägglöf, C.; Olsson, E.; Stolz, C.; Tsagozis, P.; Levchenko, T.; Frederick, M.J.; Borg, Å.; Micke, P.; Egevad, L.; et al. CXCL14 Is an Autocrine Growth Factor for Fibroblasts and Acts as a Multi-Modal Stimulator of Prostate Tumor Growth. Proc. Natl. Acad. Sci. USA 2009, 106, 3414–3419. [Google Scholar] [CrossRef] [Green Version]
- Tessema, M.; Klinge, D.M.; Yingling, C.M.; Do, K.; Van Neste, L.; Belinsky, S.A. Re-Expression of CXCL14, a Common Target for Epigenetic Silencing in Lung Cancer, Induces Tumor Necrosis. Oncogene 2010, 29, 5159–5170. [Google Scholar] [CrossRef] [Green Version]
- Hata, R.I.; Izukuri, K.; Kato, Y.; Sasaki, S.; Mukaida, N.; Maehata, Y.; Miyamoto, C.; Akasaka, T.; Yang, X.; Nagashima, Y.; et al. Suppressed Rate of Carcinogenesis and Decreases in Tumour Volume and Lung Metastasis in CXCL14/BRAK Transgenic Mice. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Karunanithi, S.; Levi, L.; DeVecchio, J.; Karagkounis, G.; Reizes, O.; Lathia, J.D.; Kalady, M.F.; Noy, N. RBP4-STRA6 Pathway Drives Cancer Stem Cell Maintenance and Mediates High-Fat Diet-Induced Colon Carcinogenesis. Stem. Cell Rep. 2017, 9, 438–450. [Google Scholar] [CrossRef] [Green Version]
- Abola, M.V.; Thompson, C.L.; Chen, Z.; Chak, A.; Berger, N.A.; Kirwan, J.P.; Li, L. Serum Levels of Retinol-Binding Protein 4 and Risk of Colon Adenoma. Endocr. Relat. Cancer 2015, 22, L1–L4. [Google Scholar] [CrossRef] [Green Version]
- Jiao, C.; Cui, L.; Ma, A.; Li, N.; Si, H. Elevated Serum Levels of Retinol-Binding Protein 4 Are Associated with Breast Cancer Risk: A Case-Control Study. PLoS ONE 2016, 11, 1–12. [Google Scholar] [CrossRef]
- El-Mesallamy, H.O.; Hamdy, N.M.; Zaghloul, A.S.; Sallam, A.M. Serum Retinol Binding Protein-4 and Neutrophil Gelatinase-Associated Lipocalin Are Interrelated in Pancreatic Cancer Patients. Scand. J. Clin. Lab. Invest. 2012, 72, 602–607. [Google Scholar] [CrossRef]
- Wang, D.D.; Zhao, Y.M.; Wang, L.; Ren, G.; Wang, F.; Xia, Z.G.; Wang, X.L.; Zhang, T.; Pan, Q.; Dai, Z.; et al. Preoperative Serum Retinol-Binding Protein 4 Is Associated with the Prognosis of Patients with Hepatocellular Carcinoma after Curative Resection. J. Cancer Res. Clin. Oncol. 2011, 137, 651–658. [Google Scholar] [CrossRef]
- Uehara, H.; Takahashi, T.; Izumi, K. Induction of Retinol-Binding Protein 4 and Placenta-Specific 8 Expression in Human Prostate Cancer Cells Remaining in Bone Following Osteolytic Tumor Growth Inhibition by Osteoprotegerin. Int. J. Oncol. 2013, 43, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Huang, W.; Wang, F.; Dai, Y.; Hu, X.; Yue, D.; Wang, S. Serum Levels of Retinol-Binding Protein 4 and the Risk of Non-Small Cell Lung Cancer: A Case-Control Study. Medicine 2020, 99, e21254. [Google Scholar] [CrossRef]
- Florea, A.; Harris, R.B.; Klimentidis, Y.C.; Kohler, L.N.; Jurutka, P.W.; Jacobs, E.T. Circulating Fibroblast Growth Factor-21 and Risk of Metachronous Colorectal Adenoma. J. Gastrointest. Cancer 2020, 173. [Google Scholar] [CrossRef]
- Qian, J.; Tikk, K.; Weigl, K.; Balavarca, Y.; Brenner, H. Fibroblast Growth Factor 21 as a Circulating Biomarker at Various Stages of Colorectal Carcinogenesis. Br. J. Cancer 2018, 119, 1374–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akyol, M.; Alacacioglu, A.; Demir, L.; Kucukzeybek, Y.; Yildiz, Y.; Gumus, Z.; Kara, M.; Salman, T.; Varol, U.; Taskaynatan, H.; et al. The Alterations of Serum FGF-21 Levels, Metabolic and Body Composition in Early Breast Cancer Patients Receiving Adjuvant Endocrine Therapy. Cancer Biomark. 2017, 18, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, Y.; Liu, M.; Wang, D.; Wang, F.; Bi, Y.; Ji, J.; Li, S.; Liu, Y.; Chen, R.; et al. Oncogenic KRAS Reduces Expression of FGF21 in Acinar Cells to Promote Pancreatic Tumorigenesis in Mice on a High-Fat Diet. Gastroenterology 2019, 157, 1413–1428.e11. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Li, X.; Luo, Y. FGF21 in Obesity and Cancer: New Insights. Cancer Lett. 2021, 499, 5–13. [Google Scholar] [CrossRef]
- Singhal, G.; Kumar, G.; Chan, S.; Fisher, F.M.; Ma, Y.; Vardeh, H.G.; Nasser, I.A.; Flier, J.S.; Maratos-Flier, E. Deficiency of Fibroblast Growth Factor 21 (FGF21) Promotes Hepatocellular Carcinoma (HCC) in Mice on a Long Term Obesogenic Diet. Mol. Metab. 2018, 13, 56–66. [Google Scholar] [CrossRef]
- Dai, H.; Hu, W.; Zhang, L.; Jiang, F.; Mao, X.; Yang, G.; Li, L. FGF21 Facilitates Autophagy in Prostate Cancer Cells by Inhibiting the PI3K–Akt–MTOR Signaling Pathway. Cell Death Dis. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Jiang, G.; Fang, J.; You, Z.; Shao, G.; Zhang, Z.; Jiao, A.; Peng, X. FGF21 Promotes Non-Small Cell Lung Cancer Progression by SIRT1/PI3K/AKT Signaling. Life Sci. 2021, 269, 118875. [Google Scholar] [CrossRef]
- Vocka, M.; Langer, D.; Fryba, V.; Petrtyl, J.; Hanus, T.; Kalousova, M.; Zima, T.; Petruzelka, L. Novel Serum Markers HSP60, CHI3L1, and IGFBP-2 in Metastatic Colorectal Cancer. Oncol. Lett. 2019, 18, 6284–6292. [Google Scholar] [CrossRef]
- Pickard, A.; McCance, D.J. IGF-Binding Protein 2—Oncogene or Tumor Suppressor? Front. Endocrinol. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Busund, L.T.R.; Richardsen, E.; Busund, R.; Ukkonen, T.; Bjørnsen, T.; Busch, C.; Stalsberg, H. Significant Expression of IGFBP2 in Breast Cancer Compared with Benign Lesions. J. Clin. Pathol. 2005, 58, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Frommer, K.W.; Reichenmiller, K.; Schutt, B.S.; Hoeflich, A.; Ranke, M.B.; Dodt, G.; Elmlinger, M.W. IGF-Independent Effects of IGFBP-2 on the Human Breast Cancer Cell Line Hs578T. J. Mol. Endocrinol. 2006, 37, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, L.; Chen, H.; Kong, R.; Pan, S.; Hu, J.; Wang, Y.; Li, Y.; Sun, B. Silencing IGFBP-2 Decreases Pancreatic Cancer Metastasis and Enhances Chemotherapeutic Sensitivity. Oncotarget 2017, 8, 61674–61686. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Cui, D.; Zhang, Y.; Han, C.C.; Wei, W. Insulin-like Growth Factor Binding Protein-2 Promotes Proliferation and Predicts Poor Prognosis in Hepatocellular Carcinoma. Onco. Targets. Ther. 2020, 13, 5083–5092. [Google Scholar] [CrossRef]
- Cohen, P.; Peehl, D.M.; Stamey, T.A.; Wilson, K.F.; Clemmons, D.R.; Rosenfeld, R.G. Elevated Levels of Insulin-like Growth Factor-Binding Protein-2 in the Serum of Prostate Cancer Patients. J. Clin. Endocrinol. Metab. 1993, 76, 1031–1035. [Google Scholar]
- Monti, S.; Di Silverio, F.; Lanzara, S.; Varasano, P.; Martini, C.; Tosti-Croce, C.; Sciarra, F. Insulin-like Growth Factor-I and -II in Human Benign Prostatic Hyperplasia: Relationship with Binding Proteins 2 and 3 and Androgens. Steroids 1998, 63, 362–366. [Google Scholar] [CrossRef]
- Yazawa, T.; Sato, H.; Shimoyamada, H.; Okudela, K.; Woo, T.; Tajiri, M.; Ogura, T.; Ogawa, N.; Suzuki, T.; Mitsui, H.; et al. Neuroendocrine Cancer-Specific up-Regulating Mechanism of Insulin-like Growth Factor Binding Protein-2 in Small Cell Lung Cancer. Am. J. Pathol. 2009, 175, 976–987. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Lu, H.; Gao, W.; Wang, L.; Lu, K.; Wu, S.; Pataer, A.; Huang, M.; El-Zein, R.; Lin, T.; et al. Insulin-Like Growth Factor Binding Protein-2 Level Is Increased in Blood of Lung Cancer Patients and Associated with Poor Survival. PLoS ONE 2013, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Shen, S.S.; Wang, H.; Diwan, A.H.; Zhang, W.; Fuller, G.N.; Prieto, V.G. Expression of Insulin-like Growth Factor-Binding Protein 2 in Melanocytic Lesions. J. Cutan. Pathol. 2003, 30, 599–605. [Google Scholar] [CrossRef]
- Giovannucci, E. Insulin, Insulin-like Growth Factors and Colon Cancer: A Review of the Evidence. J. Nutr. 2001, 131, 3109S–3120S. [Google Scholar] [CrossRef] [Green Version]
- Murphy, N.; Knuppel, A.; Papadimitriou, N.; Martin, R.M.; Tsilidis, K.K.; Smith-Byrne, K.; Fensom, G.; Perez-Cornago, A.; Travis, R.C.; Key, T.J.; et al. Insulin-like Growth Factor-1, Insulin-like Growth Factor-Binding Protein-3, and Breast Cancer Risk: Observational and Mendelian Randomization Analyses with ∼430 000 Women. Ann. Oncol. 2020, 31, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Mutgan, A.C.; Besikcioglu, H.E.; Wang, S.; Friess, H.; Ceyhan, G.O.; Demir, I.E. Insulin/IGF-Driven Cancer Cell-Stroma Crosstalk as a Novel Therapeutic Target in Pancreatic Cancer. Mol. Cancer 2018, 17, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Stuver, S.O.; Kuper, H.; Tzonou, A.; Lagiou, P.; Spanos, E.; Hsieh, C.C.; Mantzoros, C.; Trichopoulos, D. Insulin-like Growth Factor 1 in Hepatocellular Carcinoma and Metastatic Liver Cancer in Men. Int. J. Cancer 2000, 87, 118–121. [Google Scholar] [CrossRef]
- Shi, R.; Berkel, H.J.; Yu, H. Insulin-like Growth Factor-I and Prostate Cancer: A Meta-Analysis. Br. J. Cancer 2001, 85, 991–996. [Google Scholar] [CrossRef]
- Velcheti, V.; Govindan, R. Insulin-like Growth Factor and Lung Cancer. J. Thorac. Oncol. 2006, 1, 607–610. [Google Scholar]
- Nurwidya, F.; Andarini, S.; Takahashi, F.; Syahruddin, E.; Takahashi, K. Implications of Insulin-like Growth Factor 1 Receptor Activation in Lung Cancer. Malays. J. Med. Sci. 2016, 23, 9–21. [Google Scholar]
- Kucera, R.; Treskova, I.; Vrzalova, J.; Svobodova, S.; Topolcan, O.; Fuchsova, R.; Rousarova, M.; Treska, V.; Kydlicek, T. Evaluation of IGF1 Serum Levels in Malignant Melanoma and Healthy Subjects. Anticancer Res. 2014, 34, 5217–5220. [Google Scholar]
- Li, C.; Wang, J.; Kong, J.; Tang, J.; Wu, Y.; Xu, E.; Zhang, H.; Lai, M. GDF15 Promotes EMT and Metastasis in Colorectal Cancer. Oncotarget 2016, 7, 860–872. [Google Scholar] [CrossRef]
- Spanopoulou, A.; Gkretsi, V. Growth Differentiation Factor 15 (GDF15) in Cancer Cell Metastasis: From the Cells to the Patients. Clin. Exp. Metastasis 2020, 37, 451–464. [Google Scholar] [CrossRef]
- Gkretsi, V.; Louca, M.; Stylianou, A.; Minadakis, G.; Spyrou, G.M.; Stylianopoulos, T. Inhibition of Breast Cancer Cell Invasion by Ras Suppressor-1 (RSU-1) Silencing Is Reversed by Growth Differentiation Factor-15 (GDF-15). Int. J. Mol. Sci. 2019, 20, 163. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Zhang, J.; Yin, L.; Yang, J.; Zheng, Y.; Zhang, M.; Ni, B.; Wang, H. Upregulated GDF-15 Expression Facilitates Pancreatic Ductal Adenocarcinoma Progression through Orphan Receptor GFRAL. Aging 2020, 12, 22564–22577. [Google Scholar] [CrossRef]
- Liu, X.; Chi, X.; Gong, Q.; Gao, L.; Niu, Y.; Chi, X.; Cheng, M.; Si, Y.; Wang, M.; Zhong, J.; et al. Association of Serum Level of Growth Differentiation Factor 15 with Liver Cirrhosis and Hepatocellular Carcinoma. PLoS ONE 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Pang, H.L.; Chen, W.J.; Shen, W.W.; Cao, P.P.; Wang, S.M.; Liu, L.L.; Zhang, H.L. The Role of GDF15 in Bone Metastasis of Lung Adenocarcinoma Cells. Oncol. Rep. 2019, 41, 2379–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suesskind, D.; Schatz, A.; Schnichels, S.; Coupland, S.E.; Lake, S.L.; Wissinger, B.; Bartz-Schmidt, K.U.; Henke-Fahle, S. GDF-15: A Novel Serum Marker for Metastases in Uveal Melanoma Patients. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Broll, R.; Erdmann, H.; Duchrow, M.; Oevermann, E.; Schwandner, O.; Markert, U.; Bruch, H.P.; Windhövel, U. Vascular Endothelial Growth Factor (VEGF)—A Valuable Serum Tumour Marker in Patients with Colorectal Cancer? Eur. J. Surg. Oncol. 2001, 27, 37–42. [Google Scholar] [CrossRef]
- George, M.L.; Tutton, M.G.; Janssen, F.; Arnaoutz, A.; Abulafi, A.M.; Eccles, S.A.; Swift, R.I. VEGF-A, VEGF-C, and VEGF-D in Colorectal Cancer Progression. Neoplasia 2001, 3, 420–427. [Google Scholar] [CrossRef] [Green Version]
- Salven, P.; Perhoniemi, V.; Tykkä, H.; Mäenpää, H.; Joensuu, H. Serum VEGF Levels in Women with a Benign Breast Tumor or Breast Cancer. Breast Cancer Res. Treat. 1999, 53, 161–166. [Google Scholar] [CrossRef]
- Liu, Y.; Tamimi, R.M.; Collins, L.C.; Schnitt, S.J.; Gilmore, H.L.; Connolly, J.L.; Colditz, G.A. The Association between Vascular Endothelial Growth Factor Expression in Invasive Breast Cancer and Survival Varies with Intrinsic Subtypes and Use of Adjuvant Systemic Therapy: Results from the Nurses’ Health Study. Breast Cancer Res. Treat. 2009, 129, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Costache, M.I.; Ioana, M.; Iordache, S.; Ene, D.; Costache, C.A.; Săftoiu, A. VEGF Expression in Pancreatic Cancer and Other Malignancies: A Review of the Literature. Rom. J. Intern. Med. 2015, 53, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Mukozu, T.; Nagai, H.; Matsui, D.; Kanekawa, T.; Sumino, Y. Serum VEGF as a Tumor Marker in Patients with HCV-Related Liver Cirrhosis and Hepatocellular Carcinoma. Anticancer Res. 2013, 33, 1013–1022. [Google Scholar] [CrossRef]
- Botelho, F.; Pina, F.; Lunet, N. VEGF and Prostatic Cancer: A Systematic Review. Eur. J. Cancer Prev. 2010, 19, 385–392. [Google Scholar] [CrossRef]
- Frezzetti, D.; Gallo, M.; Maiello, M.R.; D’Alessio, A.; Esposito, C.; Chicchinelli, N.; Normanno, N.; De Luca, A. VEGF as a Potential Target in Lung Cancer. Expert Opin. Ther. Targets 2017, 21, 959–966. [Google Scholar] [CrossRef]
- Redondo, P.; Bandrés, E.; Solano, T.; Okroujnov, I.; García-Foncillas, J. Vascular Endothelial Growth Factor (VEGF) and Melanoma. N-Acetylcysteine Downregulates VEGF Production in Vitro. Cytokine 2000, 12, 374–378. [Google Scholar] [CrossRef]
- Bernard, J.K.; McCann, S.P.; Bhardwaj, V.; Washington, M.K.; Frey, M.R. Neuregulin-4 Is a Survival Factor for Colon Epithelial Cells Both in Culture and in Vivo. J. Biol. Chem. 2012, 287, 39850–39858. [Google Scholar] [CrossRef] [Green Version]
- Dunn, M.; Sinha, P.; Campbell, R.; Blackburn, E.; Levinson, N.; Rampaul, R.; Bates, T.; Humphreys, S.; Gullick, W.J. Co-Expression of Neuregulins 1,2,3 and 4 in Human Breast Cancer. J. Pathol. 2004, 203, 672–680. [Google Scholar] [CrossRef]
- Li, M.; Chen, Y.; Jiang, J.; Lu, Y.; Song, Z.; Zhang, S.; Sun, C.; Ying, H.; Fan, X.; Song, Y.; et al. Elevated Serum Neuregulin 4 Levels in Patients with Hyperthyroidism. Endocr. Connect. 2019, 8, 728–735. [Google Scholar] [CrossRef] [Green Version]
- Blüher, M. Neuregulin 4: A “Hotline” Between Brown Fat and Liver. Obesity 2019, 27, 1555–1557. [Google Scholar] [CrossRef] [Green Version]
- Hayes, N.V.L.; Blackburn, E.; Smart, L.V.; Boyle, M.M.; Russell, G.A.; Frost, T.M.; Morgan, B.J.T.; Baines, A.J.; Gullick, W.J. Identification and Characterization of Novel Spliced Variants of Neuregulin 4 in Prostate Cancer. Clin. Cancer Res. 2007, 13, 3147–3155. [Google Scholar] [CrossRef] [Green Version]
- Stove, C.; Stove, V.; Derycke, L.; Van Marck, V.; Mareel, M.; Bracke, M. The Heregulin/Human Epidermal Growth Factor Receptor as a New Growth Factor System in Melanoma with Multiple Ways of Deregulation. J. Invest. Dermatol. 2003, 121, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Lengyel, E.; Makowski, L.; DiGiovanni, J.; Kolonin, M.G. Cancer as a Matter of Fat: The Crosstalk between Adipose Tissue and Tumors. Trends Cancer 2018, 4, 374–384. [Google Scholar] [CrossRef]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.K.; Zollo, M.; Spano, D.; Dhawan, P.; et al. Tissue Invasion and Metastasis: Molecular, Biological and Clinical Perspectives. Semin. Cancer Biol. 2015, 35, S244–S275. [Google Scholar] [CrossRef]
- Whiteside, T.L. The Tumor Microenvironment and Its Role in Promoting Tumor Growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [Green Version]
- Peinado, H.; Lavotshkin, S.; Lyden, D. The Secreted Factors Responsible for Pre-Metastatic Niche Formation: Old Sayings and New Thoughts. Semin. Cancer Biol. 2011, 21, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiang, J. Remodeling the Microenvironment before Occurrence and Metastasis of Cancer. Int. J. Biol. Sci. 2019, 15, 105–113. [Google Scholar] [CrossRef] [Green Version]
- De Lope, L.R.; Alcíbar, O.L.; López, A.A.; Hergueta-Redondo, M.; Peinado, H. Tumour–Adipose Tissue Crosstalk: Fuelling Tumour Metastasis by Extracellular Vesicles. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373. [Google Scholar]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, Biogenesis and Function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Lin, R.; Wang, S.; Zhao, R.C. Exosomes from Human Adipose-Derived Mesenchymal Stem Cells Promote Migration through Wnt Signaling Pathway in a Breast Cancer Cell Model. Mol. Cell. Biochem. 2013, 383, 13–20. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-Derived Circulating MiRNAs Regulate Gene Expression in Other Tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Peijs, L.; Beaudry, J.L.; Jespersen, N.Z.; Nielsen, C.H.; Ma, T.; Brunner, A.D.; Larsen, T.J.; Bayarri-Olmos, R.; Prabhakar, B.S.; et al. Proteomics-Based Comparative Mapping of the Secretomes of Human Brown and White Adipocytes Reveals EPDR1 as a Novel Batokine. Cell Metab. 2019, 30, 963–975.e7. [Google Scholar] [CrossRef]
- Jung, Y.J.; Kim, H.K.; Cho, Y.; Choi, J.S.; Woo, C.H.; Lee, K.S.; Sul, J.H.; Lee, C.M.; Han, J.; Park, J.H.; et al. Cell Reprogramming Using Extracellular Vesicles from Differentiating Stem Cells into White/Beige Adipocytes. Sci. Adv. 2020, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Salomon, C.; Freeman, D.J. Extracellular Vesicles from Adipose Tissue-A Potential Role in Obesity and Type 2 Diabetes? Front. Endocrinol. 2017, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Koeck, E.S.; Iordanskaia, T.; Sevilla, S.; Ferrante, S.C.; Hubal, M.J.; Freishtat, R.J.; Nadler, E.P. Adipocyte Exosomes Induce Transforming Growth Factor Beta Pathway Dysregulation in Hepatocytes: A Novel Paradigm for Obesity-Related Liver Disease. J. Surg. Res. 2014, 192, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Kurywchak, P.; Tavormina, J.; Kalluri, R. The Emerging Roles of Exosomes in the Modulation of Immune Responses in Cancer. Genome Med. 2018, 10, 1–4. [Google Scholar] [CrossRef] [Green Version]

| Batokine/Gene | Physiological Activity | Cancer Type | Protein Levels 1 | Model | References |
|---|---|---|---|---|---|
| Adiponectin/ADIPOQ | Receptor mediated: Regulates proliferation Receptor independent: Regulates insulin sensitivity and modulates immune response | Colorectal | Low (serum) | H | [137] |
| Breast | Low (serum) | H | [138,139] | ||
| Pancreas | High (serum) Low (serum) | H | [140,141] | ||
| Liver | High (serum) | H | [142] | ||
| Prostate | Low (serum) | H | [143,144] | ||
| Lung | Low (advanced stages) | H | [145] | ||
| Melanoma | Low (serum) | H | [146] | ||
| Bone morphogenetic proteins/BMPx | Receptor mediated: Regulates proliferation, differentiation and apoptosis | Colorectal | High BMP2 (metastasis) Low BMP3 (Primary) | H | [147,148] |
| Breast | High BMP4, BMP7 (CL, Primary) | CL, M, H | [148,149] | ||
| Pancreas | Low (Cells) | CL | [150] | ||
| Liver | High (serum) | M | [151] | ||
| Prostate | Low (Primary) | H | [152] | ||
| Lung | BMP2 and BMP 4 high (Primary). BMP7 high (metastasis) | CL, H | [153,154] | ||
| Melanoma | BMP7 high (Primary) | H | [155] | ||
| C-X-C Motif Chemokine Ligand 14/CXCL-14 | Implicated in recruitment of immune cells and immune surveillance | Colorectal | Low (Primary) | H | [156] |
| Breast | Low (Primary, CL) | CL, M, H | [157,158] | ||
| Pancreas | High (Primary, CL) | CL, H | [159] | ||
| Liver | Low (Primary, CL) | CL, H | [160] | ||
| Prostate | High (Primary, CL) | CL, H | [161] | ||
| Lung | Methylated (Primary) | H | [162] | ||
| Melanoma | N/A | H | [163] | ||
| Retinol binding protein 4/RBP-4 | Regulates insulin resistance and glucose homeostasis | Colorectal | High (serum), High (CL) | CL, H | [164,165] |
| Breast | High (serum) | H | [166] | ||
| Pancreas | High (serum, Primary) | H | [167] | ||
| Liver | High (CL), Correlation serum levels-survival | CL, H | [168] | ||
| Prostate | High (CL) | CL | [169] | ||
| Lung | High (serum) | H | [170] | ||
| Melanoma | N/A | ||||
| Fibroblast growth factor 21/FGF-21 | Regulates cell proliferation, glucose homeostasis and acts as a stress sensor | Colorectal | High (serum) | H | [171,172] |
| Breast | Low (serum) with hormonal therapy | H | [173] | ||
| Pancreas | Low (Primary) | M | [174,175] | ||
| Liver | High (Primary) | M | [175,176] | ||
| Prostate | Low (Primary, CL) | CL, M, H | [177] | ||
| Lung | High (Primary, CL) | H | [178] | ||
| Melanoma | N/A | ||||
| Insulin growth factor binding protein 2/IGFBP-2 | Interacts with components of ECM, controls cell growth and metabolism | Colorectal | High (serum) | H | [179,180] |
| Breast | High (Serum, Primary) | CL, H | [181,182] | ||
| Pancreas | High (pancreatic juice, serum) | H | [183] | ||
| Liver | High (serum) | H | [180,184] | ||
| Prostate | High (serum, CL) | CL, H | [185,186] | ||
| Lung | High (serum), Secreted (CL) | CL, H | [187,188] | ||
| Melanoma | High (Primary, CL) | CL, H | [189] | ||
| Insulin like growth factor 1/IGF-1 | Displays activity by a receptor mediated interaction. Plays an important role in growth and aging | Colorectal | Correlates with cancer risk | H | [190] |
| Breast | High (serum) | H | [182,191] | ||
| Pancreas | High (Primary) | H | [192] | ||
| Liver | Low (serum) | H | [193] | ||
| Prostate | High (serum, Primary) | H | [186,194] | ||
| Lung | High (plasma) | H | [195,196] | ||
| Melanoma | High (serum) | H | [197] | ||
| Growth differentiation factor 15/GDF-15 | Important role in regulation of inflammatory pathways by inhibition of macrophages and involvement in regulation of apoptosis, cell growth and cell repair | Colorectal | High (serum, Primary) | CL, H | [198,199] |
| Breast | Suppression promotes metastasis | CL, H | [200] | ||
| Pancreas | High (plasma, Primary) | CL, H | [201] | ||
| Liver | High (Primary) | H | [202] | ||
| Prostate | High (serum) | CL, H | [199] | ||
| Lung | Correlates with metastasis | CL, M | [203] | ||
| Melanoma | High (serum) | H | [204] | ||
| Vascular endothelial growth factor/VEGF | Involved in angiogenesis and normal physiological function, development, wound healing, hematopoiesis | Colorectal | High mRNA (Primary) High (serum) | H | [205,206] |
| Breast | High (Primary), High (serum) | H | [207,208] | ||
| Pancreas | High (Primary) Correlation with TNM | H | [209] | ||
| Liver | High (serum) | H | [210] | ||
| Prostate | High (plasma, Primary) | H | [211] | ||
| Lung | High (serum) | H | [212] | ||
| Melanoma | High (serum, CL) | CL, H | [213] | ||
| Neuregulin 4/NRG-4 | Potential activity in inhibition of lipogenesis in liver and control of glucose and lipid homeostasis | Colorectal | Induces cell survival in cells | CL, M | [214] |
| Breast | High (Primary) | H | [215] | ||
| Pancreas | Normal pancreas function | H | [216] | ||
| Liver | Protection against IR | M, H | [217] | ||
| Prostate | High (advanced stages) | H | [218] | ||
| Lung | N/A | ||||
| Melanoma | High (Primary, CL) | H | [219] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Artime, A.; García-Soler, B.; Sainz, R.M.; Mayo, J.C. Emerging Roles for Browning of White Adipose Tissue in Prostate Cancer Malignant Behaviour. Int. J. Mol. Sci. 2021, 22, 5560. https://doi.org/10.3390/ijms22115560
Álvarez-Artime A, García-Soler B, Sainz RM, Mayo JC. Emerging Roles for Browning of White Adipose Tissue in Prostate Cancer Malignant Behaviour. International Journal of Molecular Sciences. 2021; 22(11):5560. https://doi.org/10.3390/ijms22115560
Chicago/Turabian StyleÁlvarez-Artime, Alejandro, Belén García-Soler, Rosa María Sainz, and Juan Carlos Mayo. 2021. "Emerging Roles for Browning of White Adipose Tissue in Prostate Cancer Malignant Behaviour" International Journal of Molecular Sciences 22, no. 11: 5560. https://doi.org/10.3390/ijms22115560