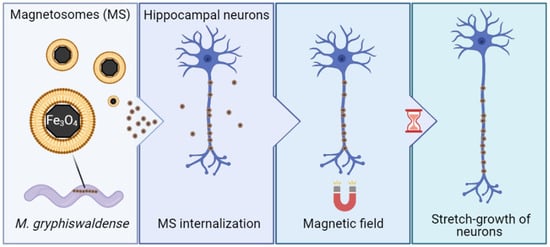
Induction of Axonal Outgrowth in Mouse Hippocampal Neurons via Bacterial Magnetosomes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Magnetosome Cell Interactions
2.2. Magnetosomes Induce Stretch-Growth
3. Materials and Methods
3.1. Animals
3.2. Cell Culture
3.3. Chemically Synthesized Nanoparticles
3.4. Cultivation of Magnetospirillum gryphiswaldense
3.5. Magnetosome Isolation and Purification
3.6. Determination of Iron Concentrations
3.7. Fluorescent Labelling of Isolated Magnetosomes
3.8. Sterile Filtration of Magnetosome Suspensions
3.9. Transmission Electron Microscopy
3.10. Analytical Methods
3.11. Bio-Synthetized versus Artificially Synthetized Nanoparticles
3.12. Magnetic Field
3.13. Stretching Assay
3.14. Toxicity Test
3.15. Particle Localization
3.16. Immunostaining
3.17. Image Analysis
3.18. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calaghan, S.C.; Belus, A.; White, E. Do stretch-induced changes in intracellular calcium modify the electrical activity of cardiac muscle? Prog. Biophys. Mol. Biol. 2003, 82, 81–95. [Google Scholar] [CrossRef]
- Stewart, S.; Darwood, A.; Masouros, S.; Higgins, C.; Ramasamy, A. Mechanotransduction in osteogenesis. Bone Jt. Res. 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Schubert, R.; Brayden, J.E. Stretch-Activated Cation Channels and the Myogenic Response of Small Arteries; Academia: Moscow, Russia, 2005; ISBN 5769525908. [Google Scholar]
- Athamneh, A.I.M.; Suter, D.M. Quantifying mechanical force in axonal growth and guidance. Front. Cell. Neurosci. 2015, 9, 359. [Google Scholar] [CrossRef] [Green Version]
- De Vincentiis, S.; Falconieri, A.; Mainardi, M.; Cappello, V.; Scribano, V.; Bizzarri, R.; Storti, B.; Dente, L.; Costa, M.; Raffa, V. Extremely Low Forces Induce Extreme Axon Growth. J. Neurosci. 2020, 40, 4997–5007. [Google Scholar] [CrossRef]
- Raffa, V.; Falcone, F.; De Vincentiis, S.; Falconieri, A.; Calatayud, M.P.; Goya, G.F.; Cuschieri, A. Piconewton Mechanical Forces Promote Neurite Growth. Biophys. J. 2018, 115, 2026–2033. [Google Scholar] [CrossRef] [Green Version]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.D.; Schüler, D.; Pfeiffer, D. A Compass to Boost Navigation: Cell Biology of Bacterial Magnetotaxis. J. Bacteriol. 2020, 202. [Google Scholar] [CrossRef]
- Jogler, C.; Schüler, D. Genomics, Genetics, and Cell Biology of Magnetosome Formation. Annu. Rev. Microbiol. 2009, 63, 501–521. [Google Scholar] [CrossRef] [Green Version]
- Uebe, R.; Schüler, D. Magnetosome biogenesis in magnetotactic bacteria. Nat. Rev. Microbiol. 2016, 14, 621–637. [Google Scholar] [CrossRef]
- Grünberg, K.; Wawer, C.; Tebo, B.M.; Schüler, D. A Large Gene Cluster Encoding Several Magnetosome Proteins Is Conserved in Different Species of Magnetotactic Bacteria. Appl. Environ. Microbiol. 2001, 67, 4573–4582. [Google Scholar] [CrossRef] [Green Version]
- Grünberg, K.; Müller, E.C.; Otto, A.; Reszka, R.; Linder, D.; Kube, M.; Reinhardt, R.; Schüler, D. Biochemical and Proteomic Analysis of the Magnetosome Membrane in Magnetospirillum gryphiswaldense. Appl. Environ. Microbiol. 2004, 70, 1040–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raschdorf, O.; Bonn, F.; Zeytuni, N.; Zarivach, R.; Becher, D.; Schüler, D. A quantitative assessment of the membrane-integral sub-proteome of a bacterial magnetic organelle. J. Proteom. 2018, 172, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenfeldt, S.; Mickoleit, F.; Jörke, C.; Clement, J.H.; Markert, S.; Jérôme, V.; Schwarzinger, S.; Freitag, R.; Schüler, D.; Uebe, R.; et al. Towards standardized purification of bacterial magnetic nanoparticles for future in vivo applications. Acta Biomater. 2021, 120, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Lohße, A.; Borg, S.; Raschdorf, O.; Kolinko, I.; Tompa, É.; Pósfai, M.; Faivre, D.; Baumgartner, J.; Schülera, D. Genetic dissection of the mamAB and mms6 operons reveals a gene set essential for magnetosome biogenesis in magnetospirillum gryphiswaldense. J. Bacteriol. 2014, 196, 2658–2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alphandéry, E.; Ngo, A.T.; Lefèvre, C.; Lisiecki, I.; Wu, L.F.; Pileni, M.P. Difference between the Magnetic Properties of the Magnetotactic Bacteria and Those of the Extracted Magnetosomes: Influence of the Distance between the Chains of Magnetosomes. J. Phys. Chem. C 2008, 112, 12304–12309. [Google Scholar] [CrossRef]
- Carvallo, C.; Sainctavit, P.; Arrio, M.A.; Menguy, N.; Wang, Y.; Ona-Nguema, G.; Brice-Profeta, S. Biogenic vs. abiogenic magnetite nanoparticles: A XMCD study. Am. Mineral. 2008, 93, 880–885. [Google Scholar] [CrossRef]
- Kumari, M.; Widdrat, M.; Tompa, É.; Uebe, R.; Schüler, D.; Pósfai, M.; Faivre, D.; Hirt, A.M. Distinguishing magnetic particle size of iron oxide nanoparticles with first-order reversal curves. J. Appl. Phys. 2014, 116, 124304. [Google Scholar] [CrossRef]
- Bennet, M.; Bertinetti, L.; Neely, R.K.; Schertel, A.; Körnig, A.; Flors, C.; Müller, F.D.; Schüler, D.; Klumpp, S.; Faivre, D. Biologically controlled synthesis and assembly of magnetite nanoparticles. Faraday Discuss. 2015, 181, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, T.; Kamiya, S. Use of magnetic particles isolated from magnetotactic bacteria for enzyme immobilization. Appl. Microbiol. Biotechnol. 1987, 26, 328–332. [Google Scholar] [CrossRef]
- Sun, J.B.; Duan, J.H.; Dai, S.L.; Ren, J.; Guo, L.; Jiang, W.; Li, Y. Preparation and anti-tumor efficiency evaluation of doxorubicin-loaded bacterial magnetosomes: Magnetic nanoparticles as drug carriers isolated from Magnetospirillum gryphiswaldense. Biotechnol. Bioeng. 2008, 101, 1313–1320. [Google Scholar] [CrossRef]
- Ceyhan, B.; Alhorn, P.; Lang, C.; Schüler, D.; Niemeyer, C.M. Semisynthetic Biogenic Magnetosome Nanoparticles for the Detection of Proteins and Nucleic Acids. Small 2006, 2, 1251–1255. [Google Scholar] [CrossRef]
- Mickoleit, F.; Schüler, D. Generation of nanomagnetic biocomposites by genetic engineering of bacterial magnetosomes. Bioinspired. Biomim. Nanobiomaterials 2019, 8, 86–98. [Google Scholar] [CrossRef] [Green Version]
- Ginet, N.; Pardoux, R.; Adryanczyk, G.; Garcia, D.; Brutesco, C.; Pignol, D. Single-Step Production of a Recyclable Nanobiocatalyst for Organophosphate Pesticides Biodegradation Using Functionalized Bacterial Magnetosomes. PLoS ONE 2011, 6, e21442. [Google Scholar] [CrossRef] [Green Version]
- Borg, S.; Hofmann, J.; Pollithy, A.; Lang, C.; Schüler, D. New vectors for chromosomal integration enable high-level constitutive or inducible magnetosome expression of fusion proteins in Magnetospirillum gryphiswaldense. Appl. Environ. Microbiol. 2014, 80, 2609–2616. [Google Scholar] [CrossRef] [Green Version]
- Borg, S.; Popp, F.; Hofmann, J.; Leonhardt, H.; Rothbauer, U.; Schüler, D. An Intracellular nanotrap redirects proteins and organelles in live bacteria. MBio 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Mickoleit, F.; Schüler, D. Generation of Multifunctional Magnetic Nanoparticles with Amplified Catalytic Activities by Genetic Expression of Enzyme Arrays on Bacterial Magnetosomes. Adv. Biosyst. 2018, 2, 1700109. [Google Scholar] [CrossRef]
- Mickoleit, F.; Altintoprak, K.; Wenz, N.L.; Richter, R.; Wege, C.; Schüler, D. Precise Assembly of Genetically Functionalized Magnetosomes and Tobacco Mosaic Virus Particles Generates a Magnetic Biocomposite. ACS Appl. Mater. Interfaces 2018, 10, 37898–37910. [Google Scholar] [CrossRef]
- Mickoleit, F.; Lanzloth, C.; Schüler, D. A Versatile Toolkit for Controllable and Highly Selective Multifunctionalization of Bacterial Magnetic Nanoparticles. Small 2020, 16, 1906922. [Google Scholar] [CrossRef]
- Alphandéry, E. Applications of Magnetosomes Synthesized by Magnetotactic Bacteria in Medicine. Front. Bioeng. Biotechnol. 2014, 2, 5. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [Green Version]
- Hergt, R.; Dutz, S.; Röder, M. Effects of size distribution on hysteresis losses of magnetic nanoparticles for hyperthermia. J. Phys. Condens. Matter 2008, 20. [Google Scholar] [CrossRef]
- Taukulis, R.; Widdrat, M.; Kumari, M.; Heinke, D.; Rumpler, M.; Tompa, É.; Faivre, D. Magnetic iron oxide nanoparticles as MRI contrast agents-a comprehensive physical and theoretical study. Magnetohydrodynamics 2015, 51, 721–747. [Google Scholar]
- Vargas, G.; Cypriano, J.; Correa, T.; Leão, P.; Bazylinski, D.; Abreu, F. Applications of Magnetotactic Bacteria, Magnetosomes and Magnetosome Crystals in Biotechnology and Nanotechnology: Mini-Review. Molecules 2018, 23, 2438. [Google Scholar] [CrossRef] [Green Version]
- Mannucci, S.; Tambalo, S.; Conti, G.; Ghin, L.; Milanese, A.; Carboncino, A.; Nicolato, E.; Marinozzi, M.R.; Benati, D.; Bassi, R.; et al. Magnetosomes Extracted from Magnetospirillum gryphiswaldense as Theranostic Agents in an Experimental Model of Glioblastoma. Contrast Media Mol. Imaging 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cypriano, J.; Werckmann, J.; Vargas, G.; Lopes dos Santos, A.; Silva, K.T.; Leão, P.; Almeida, F.P.; Bazylinski, D.A.; Farina, M.; Lins, U.; et al. Uptake and persistence of bacterial magnetite magnetosomes in a mammalian cell line: Implications for medical and biotechnological applications. PLoS ONE 2019, 14, e0215657. [Google Scholar] [CrossRef] [PubMed]
- Mickoleit, F.; Jörke, C.; Geimer, S.; Maier, D.S.; Müller, J.P.; Demut, J.; Gräfe, C.; Schüler, D.; Clement, J.H. Biocompatibility, uptake and subcellular localization of bacterial magnetosomes in mammalian cells. Nanoscale Adv. 2021. under revision. [Google Scholar]
- Shin, J.; Yoo, C.-H.; Lee, J.; Cha, M. Cell response induced by internalized bacterial magnetic nanoparticles under an external static magnetic field. Biomaterials 2012, 33, 5650–5657. [Google Scholar] [CrossRef]
- Mickoleit, F.; Jérôme, V.; Freitag, R.; Schüler, D. Bacterial Magnetosomes as Novel Platform for the Presentation of Immunostimulatory, Membrane-Bound Ligands in Cellular Biotechnology. Adv. Biosyst. 2020, 4, 1900231. [Google Scholar] [CrossRef]
- Xiang, Z.; Yang, X.; Xu, J.; Lai, W.; Wang, Z.; Hu, Z.; Tian, J.; Geng, L.; Fang, Q. Tumor detection using magnetosome nanoparticles functionalized with a newly screened EGFR/HER2 targeting peptide. Biomaterials 2017, 115, 53–64. [Google Scholar] [CrossRef]
- Schuerle, S.; Furubayashi, M.; Soleimany, A.P.; Gwisai, T.; Huang, W.; Voigt, C.; Bhatia, S.N. Genetic Encoding of Targeted Magnetic Resonance Imaging Contrast Agents for Tumor Imaging. ACS Synth. Biol. 2020, 9, 392–401. [Google Scholar] [CrossRef]
- Boucher, M.; Geffroy, F.; Prévéral, S.; Bellanger, L.; Selingue, E.; Adryanczyk-Perrier, G.; Péan, M.; Lefèvre, C.T.; Pignol, D.; Ginet, N.; et al. Genetically tailored magnetosomes used as MRI probe for molecular imaging of brain tumor. Biomaterials 2017, 121, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, S.; Ghin, L.; Conti, G.; Tambalo, S.; Lascialfari, A.; Orlando, T.; Benati, D.; Bernardi, P.; Betterle, N.; Bassi, R.; et al. Magnetic Nanoparticles from Magnetospirillum gryphiswaldense Increase the Efficacy of Thermotherapy in a Model of Colon Carcinoma. PLoS ONE 2014, 9, e108959. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tang, T.; Duan, J.; Xu, P.X.; Wang, Z.; Zhang, Y.; Wu, L.; Li, Y. Biocompatibility of bacterial magnetosomes: Acute toxicity, immunotoxicity and cytotoxicity. Nanotoxicology 2010, 4, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Lv, X.; Zhang, T.; Jia, P.; Yan, R.; Li, S.; Zou, R.; Xue, Y.; Dai, L. Cytotoxicity and genotoxicity of bacterial magnetosomes against human retinal pigment epithelium cells. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Le Fèvre, R.; Durand-Dubief, M.; Chebbi, I.; Mandawala, C.; Lagroix, F.; Valet, J.P.; Idbaih, A.; Adam, C.; Delattre, J.Y.; Schmitt, C.; et al. Enhanced antitumor efficacy of biocompatible magnetosomes for the magnetic hyperthermia treatment of glioblastoma. Theranostics 2017, 7, 4618–4631. [Google Scholar] [CrossRef] [PubMed]
- Kraupner, A.; Eberbeck, D.; Heinke, D.; Uebe, R.; Schüler, D.; Briel, A. Bacterial magnetosomes-nature’s powerful contribution to MPI tracer research. Nanoscale 2017, 9, 5788–5793. [Google Scholar] [CrossRef]
- Schultheiss, D.; Schüler, D. Development of a genetic system for Magnetospirillum gryphiswaldense. Arch. Microbiol. 2003, 179, 89–94. [Google Scholar] [CrossRef]
- Schüler, D.; Köhler, M. The isolation of a new magnetic spirillum. Zentralbl. Mikrobiol. 1992, 147, 150–151. [Google Scholar] [CrossRef]
- Heyen, U.; Schüler, D. Growth and magnetosome formation by microaerophilic Magnetospirillum strains in an oxygen-controlled fermentor. Appl. Microbiol. Biotechnol. 2003, 61, 536–544. [Google Scholar] [CrossRef]
- Lang, C.; Schüler, D. Expression of green fluorescent protein fused to magnetosome proteins in microaerophilic magnetotactic bacteria. Appl. Environ. Microbiol. 2008, 74, 4944–4953. [Google Scholar] [CrossRef] [Green Version]
- Moscardini, A.; Di Pietro, S.; Signore, G.; Parlanti, P.; Santi, M.; Gemmi, M.; Cappello, V. Uranium-free X solution: A new generation contrast agent for biological samples ultrastructure. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Schüler, D.; Uhl, R.; Bäuerlein, E. A simple light scattering method to assay magnetism in Magnetospirillum gryphiswaldense. FEMS Microbiol. Lett. 1995, 132, 139–145. [Google Scholar] [CrossRef]
- Hergt, R.; Hiergeist, R.; Zeisberger, M.; Schüler, D.; Heyen, U.; Hilger, I.; Kaiser, W.A. Magnetic properties of bacterial magnetosomes as potential diagnostic and therapeutic tools. J. Magn. Magn. Mater. 2005, 293, 80–86. [Google Scholar] [CrossRef]
- Kumari, M.; Hirt, A.M.; Uebe, R.; Schüler, D.; Tompa, É.; Pósfai, M.; Lorenz, W.; Ahrentorp, F.; Jonasson, C.; Johansson, C. Experimental mixtures of superparamagnetic and single-domain magnetite with respect to Day-Dunlop plots. Geochem. Geophys. Geosyst. 2015, 16, 1739–1752. [Google Scholar] [CrossRef] [Green Version]
- Riggio, C.; Calatayud, M.P.; Giannaccini, M.; Sanz, B.; Torres, T.E.; Fernández-Pacheco, R.; Ripoli, A.; Ibarra, M.R.; Dente, L.; Cuschieri, A.; et al. The orientation of the neuronal growth process can be directed via magnetic nanoparticles under an applied magnetic field. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1549–1558. [Google Scholar] [CrossRef]
- Popko, J.; Fernandes, A.; Brites, D.; Lanier, L.M. Automated analysis of neuronj tracing data. Cytom. Part A 2009, 75, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Bray, D. Axonal growth in response to experimentally applied mechanical tension. Dev. Biol. 1984, 102, 379–389. [Google Scholar] [CrossRef]
- Bray, D. Mechanical tension produced by nerve cells in tissue culture. J. Cell Sci. 1979, 37, 391–410. [Google Scholar]
- Smith, D.H. Stretch growth of integrated axon tracts: Extremes and exploitations. Prog. Neurobiol. 2009, 89, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Lamoureux, P.; Santiago, V.; Dennerll, T.; Buxbaum, R.; Heidemann, S. Tensile regulation of axonal elongation and initiation. J. Neurosci. 1991, 11, 1117–1125. [Google Scholar] [CrossRef]
- Abraham, J.-A.; Linnartz, C.; Dreissen, G.; Springer, R.; Blaschke, S.; Rueger, M.A.; Fink, G.R.; Hoffmann, B.; Merkel, R. Directing Neuronal Outgrowth and Network Formation of Rat Cortical Neurons by Cyclic Substrate Stretch. Langmuir 2019, 35, 7423–7431. [Google Scholar] [CrossRef]
- Chen, B.M.; Grinnell, A.D. Integrins and modulation of transmitter release from motor nerve terminals by stretch. Science 1995, 269, 1578–1580. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.; Stebbings, K.A.; Llano, D.A.; Saif, T. Stretch induced hyperexcitability of mice callosal pathway. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- De Vincentiis, S.; Falconieri, A.; Scribano, V.; Ghignoli, S.; Raffa, V. Manipulation of Axonal Outgrowth via Exogenous Low Forces. Int. J. Mol. Sci. 2020, 21, 8009. [Google Scholar] [CrossRef] [PubMed]
- Pita-Thomas, W.; Steketee, M.B.; Moysidis, S.N.; Thakor, K.; Hampton, B.; Goldberg, J.L. Promoting filopodial elongation in neurons by membrane-bound magnetic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 559–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steketee, M.B.; Moysidis, S.N.; Jin, X.-L.; Weinstein, J.E.; Pita-Thomas, W.; Raju, H.B.; Iqbal, S.; Goldberg, J.L. Nanoparticle-mediated signaling endosome localization regulates growth cone motility and neurite growth. Proc. Natl. Acad. Sci. USA 2011, 108, 19042–19047. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| SPIONs | Magnetosomes | |
|---|---|---|
| Core diameter | 75 ± 10 nm | 39.7 ± 8.1 nm |
| Hydrodynamic diameter | 100 nm | 51.2 ± 7.2 nm |
| Saturation magnetization | 59 Am2 kg−1 | 70–90 Am2 kg−1 |
| Coating | Glucuronic acid | Proteinaceous phospholipid membrane |
| Concentration for cell labelling | 3.6 µg Fe mL−1 | 1.25–2.5 µg Fe mL−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vincentiis, S.; Falconieri, A.; Mickoleit, F.; Cappello, V.; Schüler, D.; Raffa, V. Induction of Axonal Outgrowth in Mouse Hippocampal Neurons via Bacterial Magnetosomes. Int. J. Mol. Sci. 2021, 22, 4126. https://doi.org/10.3390/ijms22084126
De Vincentiis S, Falconieri A, Mickoleit F, Cappello V, Schüler D, Raffa V. Induction of Axonal Outgrowth in Mouse Hippocampal Neurons via Bacterial Magnetosomes. International Journal of Molecular Sciences. 2021; 22(8):4126. https://doi.org/10.3390/ijms22084126
Chicago/Turabian StyleDe Vincentiis, Sara, Alessandro Falconieri, Frank Mickoleit, Valentina Cappello, Dirk Schüler, and Vittoria Raffa. 2021. "Induction of Axonal Outgrowth in Mouse Hippocampal Neurons via Bacterial Magnetosomes" International Journal of Molecular Sciences 22, no. 8: 4126. https://doi.org/10.3390/ijms22084126