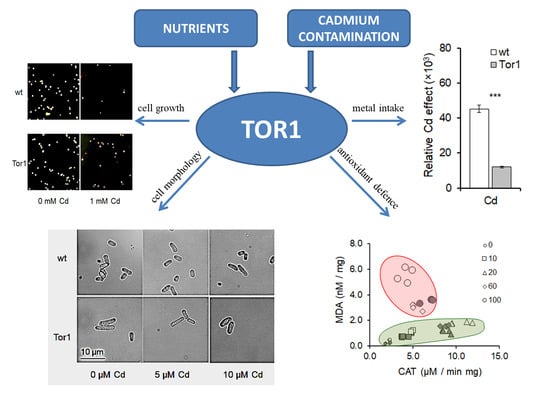
Cadmium-Induced Cell Homeostasis Impairment is Suppressed by the Tor1 Deficiency in Fission Yeast
Abstract
:1. Introduction
2. Results
2.1. Cadmium Induces Cell Growth Alterations in A Dose Dependent Manner
2.2. Tor1 Deficiency Causes Higher Tolerance of Cells to Cd
2.3. Tor1-Null Cells Show Higher Resistance Against Cd-Mediated Oxidative Stress
2.4. Effect of Cadmium Treatment on Wild-Type and Tor1-Null Cell Morphology
2.5. Ionome Disarrangement of Wild-Type and Tor1-Deficient Cells Exposed to Cd
3. Discussion
4. Materials and Methods
4.1. Yeast Strains, Media and Growth Conditions
4.2. IC50 Value Determination
4.3. Growth Rate
4.4. Immunostaining and Microscopy
4.5. Analyses of the Cell Growth Restoration after Cd Treatment
4.6. Spot Test Analyses
4.7. Biochemical Analysis
4.8. Yeast Morphology Characterization
4.9. Pre-Analytical Sample Preparation of Yeast
4.10. Ionome Quantification
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef] [Green Version]
- Frishberg, I.V. Chapter 19-Production of zinc, cadmium and their alloy powders. In Handbook of Non-Ferrous Metal Powders; Neikov, O.D., Naboychenko, S.S., Murashova, I.V., Gopienko, V.G., Frishberg, I.V., Lotsko, D.V., Eds.; Elsevier: Oxford, UK, 2009; pp. 409–422. [Google Scholar] [CrossRef]
- Andersen, O. Chapter 4-Chelation treatment during acute and chronic metal overexposures—experimental and clinical studies. In Chelation Therapy in the Treatment of Metal Intoxication; Aaseth, J., Crisponi, G., Andersen, O., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 85–252. [Google Scholar] [CrossRef]
- Begg, S.L.; Eijkelkamp, B.A.; Luo, Z.; Couñago, R.M.; Morey, J.R.; Maher, M.J.; Ong, C.-L.Y.; McEwan, A.G.; Kobe, B.; O’Mara, M.L.; et al. Dysregulation of transition metal ion homeostasis is the molecular basis for cadmium toxicity in streptococcus pneumoniae. Nat. Commun. 2015, 6, 6418. [Google Scholar] [CrossRef] [Green Version]
- Trevors, J.T.; Stratton, G.W.; Gadd, G.M. Cadmium transport, resistance, and toxicity in bacteria, algae and fungi. Can. J. Microbiol. 1986, 32, 447–464. [Google Scholar] [CrossRef]
- Rafati-Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar] [CrossRef]
- Otsubo, Y.; Yamamato, M. TOR signaling in fission yeast. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cybulski, N.; Hall, M.N. TOR complex 2: A signaling pathway of its own. Trends Biochem. Sci. 2009, 34, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Hartmuth, S.; Petersen, J. Fission yeast Tor1 functions as part of TORC1 to control mitotic entry through the stress MAPK pathway following nutrient stress. J. Cell Sci. 2009, 122, 1737–1746. [Google Scholar] [CrossRef] [Green Version]
- Bhaskar, P.T.; Hay, N. The two TORCs and Akt. Dev. Cell 2007, 12, 487–502. [Google Scholar] [CrossRef] [Green Version]
- Wood, V.; Gwilliam, R.; Rajandream, M.-A.; Lyne, M.; Lyne, R.; Stewart, A.; Sgouros, J.; Peat, N.; Hayles, J.; Baker, S.; et al. The genome sequence of schizosaccharomyces pombe. Nature 2002, 415, 871–880. [Google Scholar] [CrossRef] [Green Version]
- Weisman, R. The fission yeast TOR proteins and the rapamycin response: An unexpected tale. In TOR: Target of Rapamycin; Thomas, G., Sabatini, D.M., Hall, M.N., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2004; pp. 85–95. [Google Scholar] [CrossRef]
- Xie, J.; Wang, X.; Proud, C.G. Who does TORC2 talk to? Biochem J. 2018, 475, 1721–1738. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Maeda, T. Evolutionarily conserved regulation of TOR signalling. J. Biochem. 2013, 154, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, T.; Priya, S.; Sharma, S.K.; Andersson, S.; Jakobsson, S.; Tanghe, R.; Ashouri, A.; Rauch, S.; Goloubinoff, P.; Christen, P.; et al. Cadmium causes misfolding and aggregation of cytosolic proteins in yeast. Mol. Cell. Biol. 2017, 37, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Gardarin, A.; Chédin, S.; Lagniel, G.; Aude, J.-C.; Godat, E.; Catty, P.; Labarre, J. Endoplasmic reticulum is a major target of cadmium toxicity in yeast. Mol. Microbiol. 2010, 76, 1034–1048. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Y.; Gao, H.; Yin, J.; Huang, L. Cadmium-Induced cell killing in sacharomyces cerevisiae involves increases in intracellular NO levels. FEMS Microbiol. Lett. 2016, 363, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Petrova, V.Y.; Pisareva, E.I.; Angelov, A.I.; Kujumdzieva, A.V. Targeting genes of Cd induced oxidative stress response in yeasts. Biotechnol. Biotechnol. Equip. 2013, 27, 3716–3724. [Google Scholar] [CrossRef]
- Nair, A.R.; DeGheselle, O.; Smeets, K.; Van Kerkhove, E.; Cuypers, A. Cadmium-Induced pathologies: Where is the oxidative balance lost (or not)? Int. J. Mol. Sci. 2013, 14, 6116–6143. [Google Scholar] [CrossRef] [Green Version]
- Nemmiche, S. Oxidative signaling response to cadmium exposure. Toxicol. Sci. 2017, 156, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Abenza, J.F.; Chessel, A.; Raynaud, W.G.; Carazo-Salas, R.E. Dynamics of cell shape inheritance in fission yeast. PLoS ONE 2014, 9, e106959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, T.; Vavylonis, D. Model of fission yeast cell shape driven by membrane-bound growth factors and the cytoskeleton. PloS Comput. Biol. 2013, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. J. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zeng, R.; Zhang, M.; Guo, L. Treatment of an acute severe cadmium poisoning patient combined with multiple organ dysfunction syndromes by integrated chinese and western medicines: A case report. Chin. J. Integr. Med. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Filipič, M. Mechanisms of cadmium induced genomic instability. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2012, 733, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ren, L.; Zhou, S.; Duan, P.; Xue, J.; Chen, H.; Feng, Y.; Yue, X.; Yuan, P.; Liu, Q.; et al. Role of B-Cell Lymphoma 2 Ovarian Killer (BOK) in Acute Toxicity of Human Lung Epithelial Cells Caused by Cadmium Chloride. Med. Sci. Monitor Int. Med. J. Exp. Clin. Res. 2019, 25, 5356–5368. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Murrieta, N.H.; Rojas-Sánchez, G.R.; Reyes-Carmona, S.; Martínez-Contreras, R.D.; Martínez- Montiel, N.; Millán-Pérez-Peña, L.; Herrera-Camacho, I.P. Study of cellular processes in higher eukaryotes using the yeast schizosaccharomyces pombe as a model. Microbiol. Agric. Hum. Heal. 2015. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, C.S.; Wood, V.; Fantes, P.A. An ancient yeast for young geneticists: A primer on the schizosaccharomyces pombe model system. Genetics 2015, 201, 403–423. [Google Scholar] [CrossRef] [Green Version]
- Hayles, J.; Nurse, P. Introduction to fission yeast as a model system. Cold Spring Harb. Protoc. 2018, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Loewith, R.; Jacinto, E.; Wullschleger, S.; Lorberg, A.; Crespo, J.L.; Bonenfant, D.; Oppliger, W.; Jenoe, P.; Hall, M.N. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 2002, 10, 457–468. [Google Scholar] [CrossRef]
- Lie, S.; Banks, P.; Lawless, C.; Lydall, D.; Petersen, J. The contribution of non-essential schizosaccharomyces pombe genes to fitness in response to altered nutrient supply and target of rapamycin activity. Open Biol. 2018, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Le, Q.G.; Ishiwata-Kimata, Y.; Kohno, K.; Kimata, Y. Cadmium impairs protein folding in the endoplasmic reticulum and induces the unfolded protein response. FEMS Yeast Res. 2016, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Tamás, M.J.; Fauvet, B.; Christen, P.; Goloubinoff, P. Misfolding and aggregation of nascent proteins: A novel mode of toxic cadmium action in vivo. Curr. Genet. 2018, 64, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, N.; Krumova, E.; Stefanova, T.; Georgieva, N.; Angelova, M. The oxidative stress response of the filamentous yeast trichosporon cutaneum R57 to copper, cadmium and chromium exposure. Biotechnol. Biotechnol. Equip. 2014, 28, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, W.; Schulz, P.J.; Furst, A.; Chien, P.K. Induction of peroxisome proliferation and increase of catalase activity in yeast, candida albicans, by cadmium. Biol. Trace Elem. Res. 1995, 50, 125–133. [Google Scholar] [CrossRef]
- Romandini, P.; Tallandini, L.; Beltramini, M.; Salvato, B.; Manzano, M.; De Bertoldi, M.; Rocco, G.P. Effects of copper and cadmium on growth, superoxide dismutase and catalase activities in different yeast atrains. Comp. Biochem. Physiol. Part. C Comp. Pharmacol. 1992, 103, 255–262. [Google Scholar] [CrossRef]
- Radhakrishnan, M.V. Effect of cadmium on catalase activity in four tissues of freshwater fish heteropneustes fossilis (bloch.). Internet J. Vet. Med. 2009, 7, 1–3. [Google Scholar]
- Chen, T.; Furst, A.; Chien, P.K. The effects of cadmium and iron on catalase activities in tubifex. J. Am. Coll. Toxicol. 1994, 13, 112–120. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, Y.; Fang, Z.; Deng, Y.; Shen, Y.; Shi, P. OLE1 reduces cadmium-induced oxidative damage in saccharomyces cerevisiae. FEMS Microbiol. Lett. 2018, 365, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Rangamani, P.; Lipshtat, A.; Azeloglu, E.U.; Calizo, R.C.; Hu, M.; Ghassemi, S.; Hone, J.; Scarlata, S.; Neves, S.R.; Iyengar, R. Decoding information in cell shape. Cell 2013, 154, 1356–1369. [Google Scholar] [CrossRef] [Green Version]
- Piel, M.; Tran, P.T. Cell shape and cell division in fission yeast minireview. Curr. Biol. 2009, 19, R823–R827. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Su, J.; Liang, P. Modeling cadmium-induced endothelial toxicity using human pluripotent stem cell-derived endothelial cells. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Benoff, S.H.; Marmar, J.L.; Centola, G.M.; Hurley, I.R. Cadmium alters testicular expression of genes regulating the structure and function of the actin cytoskeleton. Fertil. Steril. 2010, 94, S62. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, H. Cadmium toxicity. Plant. Signal. Behav. 2012, 7, 345–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, J.; Nurse, P. TOR signalling regulates mitotic commitment through the stress MAP kinase pathway and the Polo and Cdc2 kinases. Nat. Cell Biol. 2007, 9, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Villar-Tajadura, M.A.; Coll, P.M.; Madrid, M.; Cansado, J.; Santos, B.; Pérez, P. Rga2 is a Rho2 GAP that regulates morphogenesis and cell integrity in s. pombe. Mol. Microbiol. 2008, 70, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Wiley, D.J.; Chen, X.; Shah, K.; Verde, F. The conserved NDR kinase Orb6 controls polarized cell growth by spatial regulation of the small GTPase Cdc42. Curr. Biol. 2009, 19, 1314–1319. [Google Scholar] [CrossRef] [Green Version]
- Kelly, F.D.; Nurse, P. Spatial control of Cdc42 activation determines cell width in fission yeast. MBoC 2011, 22, 3801–3811. [Google Scholar] [CrossRef]
- Bendezú, F.O.; Martin, S.G. Actin cables and the exocyst form two independent morphogenesis pathways in the fission yeast. MBoC 2010, 22, 44–53. [Google Scholar] [CrossRef]
- Das, M.; Drake, T.; Wiley, D.J.; Buchwald, P.; Vavylonis, D.; Verde, F. Oscillatory dynamics of Cdc42 GTPase in the control of polarized growth. Science 2012, 337, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Choong, G.; Liu, Y.; Templeton, D.M. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem.-Biol. Interact. 2014, 211, 54–65. [Google Scholar] [CrossRef]
- Ruta, L.L.; Popa, V.C.; Nicolau, I.; Danet, A.F.; Iordache, V.; Neagoe, A.D.; Farcasanu, I.C. Calcium signaling mediates the response to cadmium toxicity in saccharomyces cerevisiae cells. FEBS Lett. 2014, 588, 3202–3212. [Google Scholar] [CrossRef] [Green Version]
- Lauer Júnior, C.M.; Bonatto, D.; Mielniczki-Pereira, A.A.; Zilles Schuch, A.; Dias, J.F.; Yoneama, M.-L.; Henriques, J.A.P. The Pmr1 protein, the major yeast Ca2+-ATPase in the golgi, regulates intracellular levels of the cadmium ion. FEMS Microbiol. Lett. 2008, 285, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penner, R.; Fleig, A. The Mg2+ and Mg2+-nucleotide-regulated channel-kinase TRPM7. Handb. Exp. Pharm. 2007, 179, 313–328. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Yu, J.; Zhu, M.; Zhao, F.; Luan, S. Cadmium impairs ion homeostasis by altering K+ and Ca2+ channel activities in rice root hair cells. Plant Cell Environ. 2012, 35, 1998–2013. [Google Scholar] [CrossRef] [PubMed]
- Van Kerkhove, E.; Pennemans, V.; Swennen, Q. Cadmium and transport of ions and substances across cell membranes and epithelia. Biometals 2010, 23, 823–855. [Google Scholar] [CrossRef]
- Duracka, M.; Lukac, N.; Kacaniova, M.; Kantor, A.; Hleba, L.; Ondruska, L.; Tvrda, E. Antibiotics versus natural biomolecules: The case of in vitro induced bacteriospermia by enterococcus faecalis in rabbit semen. Molecules 2019, 24, 4329. [Google Scholar] [CrossRef] [Green Version]
- Forsburg, S.L.; Rhind, N. Basic methods for fission yeast. Yeast 2006, 23, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Very Simple IC50 Tool Kit. Available online: http://www.ic50.tk/ (accessed on 29 August 2020).
- Rabitsch, K.P.; Gregan, J.; Schleiffer, A.; Javerzat, J.-P.; Eisenhaber, F.; Nasmyth, K. Two fission yeast homologs of Drosophila Mei-S332 are required for chromosome segregation during Meiosis I and II. Curr. Biol. 2004, 14, 287–301. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar] [CrossRef]
- Pekmez, M.; Arda, N.; Hamad, İ.; Kiğ, C.; Temizkan, G. Hydrogen peroxide-induced oxidative damages in schizosaccharomyces pombe. Biologia 2008, 63, 151–155. [Google Scholar] [CrossRef]
- Garre, E.; Raginel, F.; Palacios, A.; Julien, A.; Matallana, E. Oxidative stress responses and lipid peroxidation damage are induced during dehydration in the production of dry active wine yeasts. Int. J. Food Microbiol. 2010, 136, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pozgajova, M.; Navratilova, A.; Arvay, J.; Duranova, H.; Trakovicka, A. Impact of cadmium and nickel onion homeostasis in the yeast schizosaccharomyces pombe. J. Environ. Sci. Healthpart B 2020, 55, 166–173. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Požgajová, M.; Navrátilová, A.; Šebová, E.; Kovár, M.; Kačániová, M. Cadmium-Induced Cell Homeostasis Impairment is Suppressed by the Tor1 Deficiency in Fission Yeast. Int. J. Mol. Sci. 2020, 21, 7847. https://doi.org/10.3390/ijms21217847
Požgajová M, Navrátilová A, Šebová E, Kovár M, Kačániová M. Cadmium-Induced Cell Homeostasis Impairment is Suppressed by the Tor1 Deficiency in Fission Yeast. International Journal of Molecular Sciences. 2020; 21(21):7847. https://doi.org/10.3390/ijms21217847
Chicago/Turabian StylePožgajová, Miroslava, Alica Navrátilová, Eva Šebová, Marek Kovár, and Miroslava Kačániová. 2020. "Cadmium-Induced Cell Homeostasis Impairment is Suppressed by the Tor1 Deficiency in Fission Yeast" International Journal of Molecular Sciences 21, no. 21: 7847. https://doi.org/10.3390/ijms21217847