Cell Therapies under Clinical Trials and Polarized Cell Therapies in Pre-Clinical Studies to Treat Ischemic Stroke and Neurological Diseases: A Literature Review
Abstract
:1. Introduction
2. Methods
3. General Benefits of Cell Therapies
4. Mechanisms of Cell-Based Therapies for Stroke
4.1. Direct Exchange of Damaged Neuronal Tissue and Neuronal Replacement by Administered Cells
4.2. Angiogenesis and Neuronal Remodeling
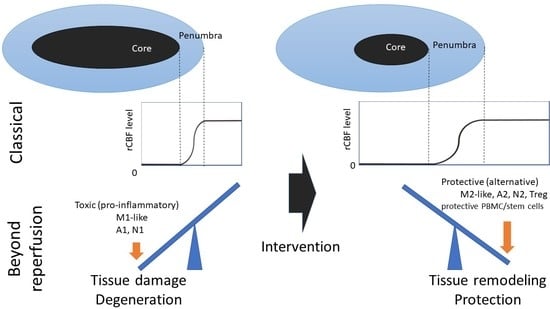
4.3. Inhibition of Inflammatory Responses
5. Cell Therapies Using BM-MSCS or BM-MNCS under Clinical Trials
6. Other Cell Sources of Cell Therapies for Cerebral Ischemia
7. Cell Therapy Using Polarized Microglia in Pre-Clinical Studies
8. Cell Therapy Using Polarized PBMCs
9. Polarization Strategies against Pathological Alterations
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AIS | acute ischemic stroke |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| BM-MNC | bone marrow mononuclear cell |
| BM-MSC | bone marrow-derived mesenchymal stem/stromal cell |
| CD | cluster of differentiation |
| CNS | central nervous system |
| DAMP | damage-associated molecular pattern |
| GDNF | glial cell-derived neurotrophic factor |
| GM-CSF | granulocyte macrophage colony-stimulating factor |
| hcMSC | human cranial bone-derived mesenchymal stem/stromal cell |
| HUCB | human umbilical cord blood |
| IL | interleukin |
| MASTERS | MultiStem® Administration for Stroke Treatment and Enhanced Recovery Study |
| MMP-9 | matrix metalloproteinase-9 |
| MNC | mononuclear cell |
| MT | mechanical thrombectomy |
| Muse | multilineage-differentiating stress-enduring |
| NSC | neuronal stem cell |
| NO | Nitrogen monoxide |
| OGD | oxygen-glucose deprivation |
| PBMC | peripheral blood mononuclear cell |
| PDGF | platelet-derived growth factor |
| rCBF | regional cerebral blood flow |
| RNAseq | RNA sequencing |
| SSEA | stage-specific embryonic antigen |
| SVZ | subventricular zone |
| TGF-β | transforming growth factor-β |
| TNF-α | tumor necrosis factor-α |
| tPA | tissue plasminogen activator |
| Treg | regulatory T cell |
| VEGF | vascular endothelial growth factor |
References
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Writing Group Members; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; et al. American Heart Association statistics committee; stroke statistics subcommittee. Executive summary: Heart disease and stroke statistics—2016 update: A report from the American Heart Association. Circulation 2016, 133, 447–454. [Google Scholar] [CrossRef]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. SWIFT PRIME Investigators. Stent–retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ye, R.; Yan, T.; Yu, S.P.; Wei, L.; Xu, G.; Fan, X.; Jiang, Y.; Stetler, R.A.; Liu, G.; et al. Cell based therapies for ischemic stroke: From basic science to bedside. Prog. Neurobiol. 2014, 115, 92–115. [Google Scholar] [CrossRef] [Green Version]
- Boese, A.C.; Le, Q.E.; Pham, D.; Hamblin, M.H.; Lee, J.P. Neural stem cell therapy for subacute and chronic ischemic stroke. Stem Cell Res. Ther. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Andres, R.H.; Horie, N.; Slikker, W.; Keren–Gill, H.; Zhan, K.; Sun, G.; Manley, N.C.; Pereira, M.P.; Sheikh, L.A.; McMillan, E.L.; et al. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 2011, 134, 1777–1789. [Google Scholar] [CrossRef]
- Chernykh, E.R.; Shevela, E.Y.; Starostina, N.M.; Morozov, S.A.; Davydova, M.N.; Menyaeva, E.V.; Ostanin, A.A. Safety and therapeutic potential of M2 macrophages in stroke treatment. Cell Transpl. 2016, 25, 1461–1471. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, M.; Miura, M.; Toriyabe, M.; Koyama, M.; Hatakeyama, M.; Ishikawa, M.; Nakajima, T.; Onodera, O.; Takahashi, T.; Nishizawa, M.; et al. Microglia preconditioned by oxygen-glucose deprivation promote functional recovery in ischemic rats. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Hatakeyama, M.; Kanazawa, M.; Ninomiya, I.; Omae, K.; Kimura, Y.; Takahashi, T.; Onodera, O.; Fukushima, M.; Shimohata, T. A novel therapeutic approach using peripheral blood mononuclear cells preconditioned by oxygen-glucose deprivation. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, M.; Takahashi, T.; Nishizawa, M.; Shimohata, T. Therapeutic strategies to attenuate hemorrhagic transformation after tissue plasminogen activator treatment for acute ischemic stroke. J. Atheroscler. Thromb. 2017, 24, 240–253. [Google Scholar] [CrossRef] [Green Version]
- Jin, K.L.; Mao, X.O.; Greenberg, A. Vascular endothelial growth factor: Direct neuroprotective effect in in vitro ischemia. Proc. Natl. Acad. Sci. USA 2000, 97, 10242–10247. [Google Scholar] [CrossRef] [Green Version]
- Jin, K.L.; Mao, X.O.; Greenberg, A. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J. Neurobiol. 2006, 66, 236–242. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, K.; Xie, L.; Childs, J.; Mao, X.O.; Logvinova, A.; Greenberg, D.A. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Investig. 2003, 111, 1843–1851. [Google Scholar] [CrossRef]
- Kanazawa, M.; Takahashi, T.; Ishikawa, M.; Onodera, O.; Shimohata, T.; del Zoppo, G.J. Angiogenesis in the ischemic core: A potential treatment target? J. Cereb. Blood Flow Metab. 2019, 39, 753–769. [Google Scholar] [CrossRef]
- Kanazawa, M.; Igarashi, H.; Kawamura, K.; Takahashi, T.; Kakita, A.; Takahashi, H.; Nakada, T.; Nishizawa, M.; Shimohata, T. Inhibition of VEGF signaling pathway attenuates hemorrhage after tPA treatment. J. Cereb. Blood Flow Metab. 2011, 31, 1461–1474. [Google Scholar] [CrossRef]
- Yang, R.; Bunting, S.; Ko, A.; Keyt, B.A.; Modi, N.B.; Zioncheck, T.F.; Ferrara, N.; Jin, H. Substantially attenuated hemodynamic responses to Escherichia coli-derived vascular endothelial growth factor given by intravenous infusion compared with bolus injection. J. Pharmacol. Exp. Ther. 1998, 284, 103–110. [Google Scholar]
- Geiseler, S.J.; Morland, C. The Janus face of VEGF in stroke. Int. J. Mol. Sci. 2018, 19, 1362. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–25. [Google Scholar] [CrossRef]
- Sergijenko, A.; Langford–Smith, A.; Liao, A.Y.; Pickford, C.E.; McDermott, J.; Nowinski, G.; Langford-Smith, K.J.; Merry, C.L.; Jones, S.A.; Wraith, J.E.; et al. Myeloid/Microglial driven autologous hematopoietic stem cell gene therapy corrects a neuronopathic lysosomal disease. Mol. Ther. 2013, 21, 1938–1949. [Google Scholar] [CrossRef] [Green Version]
- Ahn, G.O.; Tseng, D.; Liao, C.H.; Dorie, M.J.; Czechowicz, A.; Brown, J.M. Inhibition of Mac–1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc. Natl. Acad. Sci. USA 2010, 107, 8363–8368. [Google Scholar] [CrossRef] [Green Version]
- Robin, A.M.; Zhang, Z.G.; Wang, L.; Zhang, R.L.; Katakowski, M.; Zhang, L.; Wang, Y.; Zhang, C.; Chopp, M. Stromal cell-derived factor 1a mediates neural progenitor cell motility after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2006, 26, 125–134. [Google Scholar]
- Wang, L.; Li, Y.; Chen, J.; Gautam, S.C.; Zhang, Z.; Lu, M.; Chopp, M. Ischemic cerebral tissue and MCP–1 enhance rat bone marrow stromal cell migration in interface culture. Exp. Hematol. 2002, 30, 831–836. [Google Scholar] [CrossRef]
- Hughes, P.M.; Allegrini, P.R.; Rudin, M.; Perry, V.H.; Mir, A.K.; Wiessner, C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J. Cereb. Blood Flow Metab. 2002, 22, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Oki, K.; Tatarishvili, J.; Wood, J.; Koch, P.; Wattananit, S.; Mine, Y.; Monni, E.; Tornero, D.; Ahlenius, H.; Ladewig, J.; et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells 2012, 30, 1120–1133. [Google Scholar] [CrossRef]
- Nakano-Doi, A.; Nakagomi, T.; Fujikawa, M.; Nakagomi, N.; Kubo, S.; Lu, S.; Yoshikawa, H.; Soma, T.; Taguchi, A.; Matsuyama, T. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem Cells 2010, 28, 1292–1302. [Google Scholar] [CrossRef]
- Bacigaluppi, M.; Pluchino, S.; Peruzzotti-Jametti, L.; Kilic, E.; Kilic, U.; Salani, G.; Brambilla, E.; West, M.J.; Comi, G.; Martino, G.; et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain 2009, 132, 2239–2251. [Google Scholar] [CrossRef] [Green Version]
- Del Zoppo, G.J. The neurovascular unit in the setting of stroke. J. Intern. Med. 2010, 267, 156–171. [Google Scholar] [CrossRef] [Green Version]
- Hatakeyama, M.; Ninomiya, I.; Kanazawa, M. Angiogenesis and neuronal remodeling after ischemic stroke. Neural. Regen. Res. 2020, 15, 16–19. [Google Scholar] [CrossRef]
- Teng, H.; Zhang, Z.G.; Wang, L.; Zhang, R.L.; Zhang, L.; Morris, D.; Gregg, S.R.; Wu, Z.; Jiang, A.; Lu, M.; et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J. Cereb. Blood Flow Metab. 2008, 28, 764–771. [Google Scholar] [CrossRef]
- Lei, W.L.; Xing, S.G.; Deng, C.Y.; Ju, X.C.; Jiang, X.Y.; Luo, Z.G. Laminin/β1 integrin signal triggers axon formation by promoting microtubule assembly and stabilization. Cell Res. 2012, 22, 954–972. [Google Scholar] [CrossRef] [Green Version]
- Ruan, L.; Wang, B.; ZhuGe, Q.; Jin, K. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res. 2015, 1623, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Uwamori, H.; Higuchi, T.; Arai, K.; Sudo, R. Integration of neurogenesis and angiogenesis models for constructing a neurovascular tissue. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Jin, K.; Minami, M.; Lan, J.Q.; Mao, X.O.; Batteur, S.; Simon, R.P.; Greenberg, D.A. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. USA 2001, 98, 4710–4715. [Google Scholar] [CrossRef] [Green Version]
- Grade, S.; Weng, Y.C.; Snapyan, M.; Kriz, J.; Malva, J.O.; Saghatelyan, A. Brain derived neurotrophic factor promotes vasculature-associated migration of neuronal precursors toward the ischemic striatum. PLoS ONE 2013, 8, e55039. [Google Scholar] [CrossRef] [Green Version]
- Fujioka, T.; Kaneko, N.; Ajioka, I.; Nakaguchi, K.; Omata, T.; Ohba, H.; Fässler, R.; García-Verdugo, J.M.; Sekiguchi, K.; Matsukawa, N.; et al. β1 integrin signaling promotes neuronal migration along vascular scaffolds in the post-stroke brain. EBioMedicine 2017, 16, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Benakis, C.; Bonilla, L.G.; Iadecola, C.; Anrather, J. The role of microglia and myeloid immune cells in acute cerebral ischemia. Front. Cell Neurosci. 2015, 8, 461. [Google Scholar] [CrossRef]
- Kanazawa, M.; Ninomiya, I.; Hatakeyama, M.; Takahashi, T.; Shimohata, T. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int. J. Mol. Sci. 2017, 18, 2135. [Google Scholar] [CrossRef]
- Li, J.; Zhu, H.; Liu, Y.; Li, Q.; Lu, S.; Feng, M.; Xu, Y.; Huang, L.; Ma, C.; An, Y.; et al. Human mesenchymal stem cell transplantation protects against cerebral ischemic injury and upregulates interleukin-10 expression in Macaca fascicularis. Brain Res. 2010, 1334, 65–72. [Google Scholar] [CrossRef]
- Yoo, S.W.; Chang, D.Y.; Lee, H.S.; Kim, G.H.; Park, J.S.; Ryu, B.Y.; Joe, E.H.; Lee, Y.D.; Kim, S.S.; Suh-Kim, H. Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by TGF-β. Neurobiol. Dis. 2013, 58, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Honmou, O.; Houkin, K.; Matsunaga, T.; Niitsu, Y.; Ishiai, S.; Onodera, R.; Waxman, S.G.; Kocsis, J.D. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 2011, 134, 1790–1807. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, G.K.; Kondziolka, D.; Wechsler, L.R.; Lunsford, L.D.; Coburn, M.L.; Billigen, J.B.; Kim, A.S.; Johnson, J.N.; Bates, D.; King, B.; et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: A phase 1/2a study. Stroke 2016, 4, 1817–1824. [Google Scholar] [CrossRef] [Green Version]
- Shichinohe, H.; Kawabori, M.; Iijima, H.; Teramoto, T.; Abumiya, T.; Nakayama, N.; Kazumata, K.; Terasaka, S.; Arato, T.; Houkin, K. Research on advanced intervention using novel bone marrOW stem cell (RAINBOW): A study protocol for a phase I, open–label, uncontrolled, dose–response trial of autologous bone marrow stromal cell transplantation in patients with acute ischemic stroke. BMC Neurol. 2017, 17, 179. [Google Scholar] [CrossRef]
- Niizuma, K.; Borlongan, C.V.; Tominaga, T. Application of muse cell therapy to stroke. Adv. Exp. Med. Biol. 2018, 1103, 167–186. [Google Scholar] [CrossRef]
- Hess, D.C.; Wechsler, L.R.; Clark, W.M.; Savitz, S.I.; Ford, G.A.; Chiu, D.; Yavagal, D.R.; Uchino, K.; Liebeskind, D.S.; Auchus, A.P.; et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): A randomised, double–blind, placebo–controlled, phase 2 trial. Lancet Neurol. 2017, 16, 360–368. [Google Scholar] [CrossRef]
- Taguchi, A.; Sakai, C.; Soma, T.; Kasahara, Y.; Stern, D.M.; Kajimoto, K.; Ihara, M.; Daimon, T.; Yamahara, K.; Doi, K.; et al. Intravenous autologous bone marrow mononuclear cell transplantation for stroke: Phase 1/2a clinical trial in a homogeneous group of stroke patients. Stem Cells Dev. 2015, 24, 2207–2218. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi-Taura, A.; Okinaka, Y.; Takeuchi, Y.; Ogawa, Y.; Maeda, M.; Kataoka, Y.; Gul, S.; Claussen, C.; Boltze, J.; Taguchi, A. Bone marrow mononuclear cells activate angiogenesis via gap junction-mediated cell-cell interaction. Stroke 2020, 51, 1279–1289. [Google Scholar] [CrossRef]
- Prasad, K.; Sharma, A.; Garg, A.; Mohanty, S.; Bhatnagar, S.; Johri, S.; Singh, K.K.; Nair, V.; Sarkar, R.S.; Gorthi, S.P.; et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: A multicentric, randomized trial. Stroke 2014, 45, 3618–3624. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Sane, H.; GokulChandran, N.; Khopkar, D.; Paranjape, A.; Sundaram, J.; Gandhi, S.; Badhe, P. Autologous bone marrow mononuclear cells intrathecal transplantation in chronic stroke. Stroke Res. Treat. 2014, 2014, 234095. [Google Scholar] [CrossRef] [Green Version]
- Savitz, S.I.; Misra, V.; Kasam, M.; Juneja, H.; Cox, C.S., Jr.; Alderman, S.; Aisiku, I.; Kar, S.; Gee, A.; Grotta, J.C. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann. Neurol. 2011, 70, 59–69. [Google Scholar] [CrossRef]
- Vahidy, F.S.; Haque, M.E.; Rahbar, M.H.; Zhu, H.; Rowan, P.; Aisiku, I.P.; Lee, D.A.; Juneja, H.S.; Alderman, S.; Barreto, A.D.; et al. Intravenous bone marrow mononuclear cells for acute ischemic stroke: Safety, feasibility, and effect size from a phase I clinical trial. Stem Cells 2019, 37, 1481–1491. [Google Scholar] [CrossRef]
- Ghali, A.A.; Yousef, M.K.; Ragab, O.A.; ElZamarany, E.A. Intra-arterial infusion of autologous bone marrow mononuclear stem cells in subacute ischemic stroke oatients. Front. Neurol. 2016, 7, 228. [Google Scholar] [CrossRef]
- Friedrich, M.A.G.; Martins, M.P.; Araújo, M.D.; Klamt, C.; Vedolin, L.; Garicochea, B.; Raupp, E.F.; Sartori El Ammar, J.; Machado, D.C.; Costa, J.C.; et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transpl. 2012, 21, S13–S21. [Google Scholar] [CrossRef]
- Oshita, J.; Okazaki, T.; Mitsuhara, T.; Imura, T.; Nakagawa, K.; Otsuka, T.; Kurose, T.; Tamura, T.; Abiko, M.; Takeda, M.; et al. Early transplantation of human cranial bone-derived mesenchymal stem cells enhances functional recovery in ischemic stroke model rats. Neurol. Med. Chir. 2020, 60, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Womble, T.A.; Green, S.; Shahaduzzaman, M.; Grieco, J.; Sanberg, P.R.; Pennypacker, K.R.; Willing, A.E. Monocytes are essential for the neuroprotective effect of human cord blood cells following middle cerebral artery occlusion in rat. Mol. Cell Neurosci. 2014, 59, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Vendrame, M.; Gemma, C.; Mesquita, D.; Collier, L.; Bickford, P.C.; Sanberg, C.D.; Sanberg, P.R.; Pennypacker, K.R.; Willing, A.E. Anti-inflammatory effects of human cord blood cells in a rat model of stroke. Stem Cell Dev. 2005, 14, 595–604. [Google Scholar] [CrossRef]
- Wakao, S.; Kushida, Y.; Dezawa, M. Basic characteristics of muse cells. Adv. Exp. Med. Biol. 2018, 1103, 13–41. [Google Scholar] [CrossRef]
- Poon, C.C.; Sarkar, S.; Yong, V.W.; Kelly, J. Glioblastoma–associated microglia and macrophages: Targets for therapies to improve prognosis. Brain 2017, 140, 1548–1560. [Google Scholar] [CrossRef] [Green Version]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [Green Version]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Perego, C.; Fumagalli, S.; de Simoni, M.G. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J. Neuroinflamm. 2011, 8, 174. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, M.; Kawamura, K.; Takahashi, T.; Miura, M.; Tanaka, Y.; Koyama, M.; Toriyabe, M.; Igarashi, H.; Nakada, T.; Nishihara, M.; et al. Multiple therapeutic effects of progranulin on experimental acute ischaemic stroke. Brain 2015, 138, 1932–1948. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, J.; Yu, L.; Ou, C.; Liu, X.; Zhao, X.; Wang, J. Comparison of the therapeutic effects of bone marrow mononuclear cells and microglia for permanent cerebral ischemia. Behav. Brain Res. 2013, 250, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Ge, R.; Tornero, D.; Hirota, M.; Monni, E.; Laterza, C.; Lindvall, O.; Kokaia, Z. Choroid plexus–cerebrospinal fluid route for monocyte–derived macrophages after stroke. J. Neuroinflamm. 2017, 14, 1–15. [Google Scholar] [CrossRef]
- Jin, Q.; Cheng, J.; Liu, Y.; Wu, J.; Wang, X.; Wei, S.; Zhou, X.; Qin, Z.; Jia, J.; Zhen, X. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav. Immun. 2014, 40, 131–142. [Google Scholar] [CrossRef]
- Lively, S.; Hutchings, S.; Schlichter, L.C. Molecular and cellular responses to interleukin-4 treatment in a rat model of transient ischemia. J. Neuropathol. Exp. Neurol. 2016, 75, 1058–1071. [Google Scholar] [CrossRef] [Green Version]
- Del Zoppo, G.J.; Frankowski, H.; Gu, Y.H.; Osada, T.; Kanazawa, M.; Milner, R.; Wang, X.; Hosomi, N.; Mabuchi, T.; Koziol, J.A. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. J. Cereb. Blood Flow Metab. 2012, 32, 919–932. [Google Scholar] [CrossRef] [Green Version]
- Ransohoff, R.M. A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci. 2016, 19, 987–991. [Google Scholar] [CrossRef]
- Crane, M.J.; Daley, J.M.; van Houtte, O.; Brancato, S.K.; Henry, W.L., Jr.; Albina, J.E. The monocyte to macrophage transition in the murine sterile wound. PLoS ONE 2014, 9, e86660. [Google Scholar] [CrossRef] [Green Version]
- Ukai, R.; Honmou, O.; Harada, K.; Houkin, K.; Hamada, H.; Kocsis, J.D. Mesenchymal stem cells derived from peripheral blood protects against ischemia. J. Neurotrauma 2007, 24, 508–520. [Google Scholar] [CrossRef] [Green Version]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef] [Green Version]
- Okada, S.; Nakamura, M.; Katoh, H.; Miyao, T.; Shimazaki, T.; Ishii, K.; Yamane, J.; Yoshimura, A.; Iwamoto, Y.; Toyama, Y.; et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 2006, 12, 829–834. [Google Scholar] [CrossRef]
- Hara, M.; Kobayakawa, K.; Ohkawa, Y.; Kumamaru, H.; Yokota, K.; Saito, T.; Kijima, K.; Yoshizaki, S.; Harimaya, K.; Nakashima, Y.; et al. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin–N–cadherin pathway after spinal cord injury. Nat. Med. 2017, 23, 818–828. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Jiang, D.; Gong, F.; Ge, X.; Lv, C.; Huang, C.; Feng, S.; Zhou, Z.; Rong, Y.; Wang, J.; Ji, C.; et al. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J. Nanobiotechnol. 2020, 18, 1–20. [Google Scholar] [CrossRef]
- Yun, S.P.; Kam, T.I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.S.; Karuppagounder, S.S.; Park, H.; Kim, S.; Oh, N.; et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef]
- García–Culebras, A.; Durán–Laforet, V.; Peña–Martínez, C.; Moraga, A.; Ballesteros, I.; Cuartero, M.I.; de la Parra, J.; Palma-Tortosa, S.; Hidalgo, A.; Corbí, A.L.; et al. Role of TLR4 (toll–like receptor 4) in N1/N2 neutrophil programming after stroke. Stroke 2019, 50, 2922–2932. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Komai, K.; Mise–Omata, S.; Iizuka–Koga, M.; Noguchi, Y.; Kondo, T.; Sakai, R.; Matsuo, K.; Nakayama, T.; Yoshie, O.; et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 2019, 565, 246–250. [Google Scholar] [CrossRef]
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Savitz, S.I.; Cramer, S.C.; Wechsler, L. STEPS 3 Consortium. Stem cells as an emerging paradigm in stroke 3: Enhancing the development of clinical trials. Stroke 2014, 45, 634–639. [Google Scholar] [CrossRef] [Green Version]
- Astrup, J.; Siesjö, B.K.; Symon, L. Thresholds in cerebral ischemia–the ischemic penumbra. Stroke 1981, 12, 723–725. [Google Scholar] [CrossRef] [Green Version]



| Autologous Cells | Allogenic Cells | ||
|---|---|---|---|
| Research Name Initiative | Cell Type (Product Name) Markers | Research Name Initiative | Cell Type (Product Name) Markers |
| INVEST-CI study Initiative: Sapporo Medical University [42] | Bone marrow-derived mesenchymal cells (STR01) CD34 (−) CD45 (−) CD105 (+) Ongoing | Initiative: SanBio company limited [43] NCT02448641 | Notch1-transfected bone marrow-derived cells (SB623) CD29 (+) CD90 (+) CD105 (+) CD34 (−) CD45 (−) No beneficial effect |
| RAINBOW study Initiative: Hokkaido University [44] | Bone marrow-derived stromal cells (HUNS001) CD34 (−) CD45 (−) CD105 (+) Ongoing | Initiative: Tohoku University [45] | Bone marrow-derived cells CD34 (−) CD45 (−) SSEA-1 (+) Ongoing |
| MASTERS study Initiative: Athersys, Inc., Healios K.K. [46] | Bone marrow-derived cells CD34 (−) CD45 (−) SSEA-1 (+) Ongoing | ||
| Reference | ClinicalTrials.gov Identifier | Source | Study Design | Comments |
|---|---|---|---|---|
| Taguchi, et al. Stem Cell Dev 2015 [47], Kikuchi-Taura et al. Stroke 2020 [48] | NCT01028794 | Autologous bone marrow mononuclear cell (CD34+) | Phase Ⅰ/Ⅱa | Improving outcome |
| Prasad, et al. Stroke 2014 [49] | NCT01501773 | Autologous bone marrow mononuclear cell | Phase Ⅱ | No beneficial effect |
| Sharma, et al. Stroke Res Treat 2014 [50] | NCT02065778 | Autologous bone marrow mononuclear cell | Phase Ⅰ | Improving outcome |
| NCT00950521 | Autologous peripheral blood stem cell CD34+) | Phase Ⅱ | No study results | |
| Savitz et al. Ann Neurol 2011 [51] Vahidy et al. Stem Cells 2019 [52] | NCT00859014 | Autologous bone marrow mononuclear cell | Phase Ⅰ | Safety |
| NCT00473057 | Autologous bone marrow cell | Phase Ⅰ | No study results | |
| Ghali, et al. Front Neurol 2016 [53] | - | Autologous bone marrow cell | Open | No beneficial effect |
| Chernykh, et al. Cell Transplant 2016 [8] | - | Autologous blood mononuclear cell (CD14+) | Open | Improving outcome |
| Friedrich, et al. Cell Transplant 2012 [54] | - | Autologous bone marrow mononuclear cell | Open | Improving outcome |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatakeyama, M.; Ninomiya, I.; Otsu, Y.; Omae, K.; Kimura, Y.; Onodera, O.; Fukushima, M.; Shimohata, T.; Kanazawa, M. Cell Therapies under Clinical Trials and Polarized Cell Therapies in Pre-Clinical Studies to Treat Ischemic Stroke and Neurological Diseases: A Literature Review. Int. J. Mol. Sci. 2020, 21, 6194. https://doi.org/10.3390/ijms21176194
Hatakeyama M, Ninomiya I, Otsu Y, Omae K, Kimura Y, Onodera O, Fukushima M, Shimohata T, Kanazawa M. Cell Therapies under Clinical Trials and Polarized Cell Therapies in Pre-Clinical Studies to Treat Ischemic Stroke and Neurological Diseases: A Literature Review. International Journal of Molecular Sciences. 2020; 21(17):6194. https://doi.org/10.3390/ijms21176194
Chicago/Turabian StyleHatakeyama, Masahiro, Itaru Ninomiya, Yutaka Otsu, Kaoru Omae, Yasuko Kimura, Osamu Onodera, Masanori Fukushima, Takayoshi Shimohata, and Masato Kanazawa. 2020. "Cell Therapies under Clinical Trials and Polarized Cell Therapies in Pre-Clinical Studies to Treat Ischemic Stroke and Neurological Diseases: A Literature Review" International Journal of Molecular Sciences 21, no. 17: 6194. https://doi.org/10.3390/ijms21176194