Long-Term Lead Exposure Since Adolescence Causes Proteomic and Morphological Alterations in the Cerebellum Associated with Motor Deficits in Adult Rats
Abstract
:1. Introduction
2. Results
2.1. The Cerebellar Damage Caused by Pb Exposure is Associated with Increased Levels of the Metal in Cerebellar Parenchyma of Adult Rats
2.2. Long-Term Exposure to Pb Since Adolescence Induced Cerebellar-Related Motor Impairments in the Adulthood.
2.3. Long-Term Exposure to Pb Induced Cerebellar Tissue Damage of Rats
2.4. Long-Term Exposure to Pb is not Associated with Oxidative Stress Triggering
2.5. Long-Term Exposure to Pb Modulates Significantly the Cerebellum Proteomic Profile of Rats
3. Discussion
4. Materials and Methods
4.1. Ethic Statement
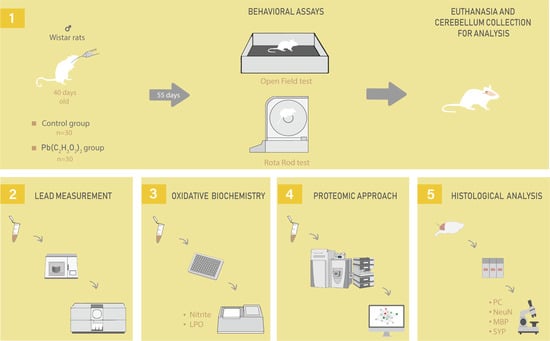
4.2. Animals and Experimental Design
4.3. Behavioral Assay
4.3.1. Open Field
4.3.2. Forced Locomotion in Gyratory Cylinder (Rota-rod)
4.4. Fresh Samples Collection
4.5. Pb Levels Quantification
4.6. Oxidative Biochemistry Analyses
4.6.1. Assay of Nitrite Levels
4.6.2. Analysis of Lipid Peroxidation (LPO) by Malondialdehyde (MDA) levels
4.7. Proteomic Approach
4.7.1. Proteomic Analysis: Preparation of the Samples
4.7.2. Mass Spectrometry analyses
4.8. Perfusion and Histological Procedures
4.8.1. Purkinje Cell Counting
4.8.2. Immunohistochemical Assays
4.8.3. Quantitative Analyses of Immunohistochemistry Assays
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AP | Action potentiation |
| BBB | Blood-brain barrier |
| CNS | Central Nervous System |
| GO | Gene Ontology |
| HE | Hematoxylin-Eosin |
| MBP | Myelin basic protein |
| MDA | Malondialdehyde |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MS | Myelin sheath |
| OF | Open field |
| Pb | Lead |
| PC | Purkinje cells |
| SYN | Synaptic vesicles |
| WHO | World Health Organization |
| MDPI | Multidisciplinary Digital Publishing Institute |
| Pb | Lead |
References
- Thürmer, K.; Williams, E.; Reutt-Robey, J. Autocatalytic Oxidation of Lead Crystallite Surfaces. Science 2002, 297, 2033–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortoul, T.I.; Moncada-Hernandez, S.; Saldivar-Osorio, L.; Espejel-Maya, G.; Mussali-Galante, P.; del Carmen Avila-Casado, M.; Colin-Barenque, L.; Hernandez-Serrato, M.I.; Avila-Costa, M.R. Sex differences in bronchiolar epithelium response after the inhalation of lead acetate (Pb). Toxicology 2005, 207, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Abadin, H.; Ashizawa, A.; Stevens, Y.W.; Llados, F.; Diamond, G.; Sage, G.; Citra, M.; Quinones, A.; Bosch, S.J.; Swarts, S.G. Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. In Toxicological Profile for Lead; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2007. [Google Scholar]
- Coakley, J. Environmental Health Criteria—Inorganic Lead. Pathology 1997, 29, 247–248. [Google Scholar] [CrossRef]
- Soliman, A.M.; Abu-Basha, E.A.; Youssef, S.A.H.; Amer, A.M.; Murphy, P.A.; Hauck, C.C.; Gehring, R.; Hsu, W.H. Effect of chronic lead intoxication on the distribution and elimination of amoxicillin in goats. J. Vet. Sci. 2013, 14, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017.
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Mudipalli, A. Lead hepatotoxicity & potential health effects. Indian J. Med Res. 2008, 126, 518–527. [Google Scholar]
- Needleman, H.L. The future challenge of lead toxicity. Environ. Health Perspect. 1990, 89, 85–89. [Google Scholar] [CrossRef]
- Stewart, W.F.; Schwartz, B.S.; Davatzikos, C.; Shen, D.; Liu, D.; Wu, X.; Todd, A.C.; Shi, W.; Bassett, S.; Youssem, D. Past adult lead exposure is linked to neurodegeneration measured by brain MRI. Neurology 2006, 66, 1476–1484. [Google Scholar] [CrossRef]
- Cecil, K.M.; Brubaker, C.J.; Adler, C.M.; Dietrich, K.N.; Altaye, M.; Egelhoff, J.C.; Wessel, S.; Elangovan, I.; Hornung, R.; Jarvis, K.; et al. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008, 5, e112. [Google Scholar] [CrossRef]
- Jusko, T.A.; Henderson, C.R.; Lanphear, B.P.; Cory-Slechta, D.A.; Parsons, P.J.; Canfield, R.L. Blood lead concentrations <10 microg/dL and child intelligence at 6 years of age. Environ. Health Perspect. 2008, 116, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Bosiacka, I.; Gutowska, I.; Rybicka, M.; Nowacki, P.; Chlubek, D. Neurotoxicity of lead. Hypothetical molecular mechanisms of synaptic function disorders. Neurologia i Neurochirurgia Polska 2012, 46, 569–578. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Nester, M.; Mashhour, A.; Moncari, L.; Shinwari, N.; Mohamed Gel, D.; Rabah, A. Prenatal and postnatal lead exposure and early cognitive development: Longitudinal study in Saudi Arabia. J. Environ. Pathol. Toxicol. Oncol. 2009, 28, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Bijoor, A.R.; Sudha, S.; Venkatesh, T. Neurochemical and neurobehavioral effects of low lead exposure on the developing brain. Indian J. Clin. Biochem. 2012, 27, 147–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradbury, M.W.; Deane, R. Permeability of the blood-brain barrier to lead. Neurotoxicology 1993, 14, 131–136. [Google Scholar]
- Evarts, E.V.; Thach, W.T. Motor mechanisms of the CNS: Cerebrocerebellar interrelations. Annu. Rev. Physiol. 1969, 31, 451–498. [Google Scholar] [CrossRef]
- Kemp, J.M.; Powell, T.P. The termination of fibres from the cerebral cortex and thalamus upon dendritic spines in the caudate nucleus: A study with the Golgi method. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1971, 262, 429–439. [Google Scholar] [CrossRef]
- Strick, P.L. How do the basal ganglia and cerebellum gain access to the cortical motor areas? Behav. Brain Res. 1985, 18, 107–123. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Pandya, D.N. The cerebrocerebellar system. Int. Rev. Neurobiol. 1997, 41, 31–60. [Google Scholar] [CrossRef]
- Gomi, H.; Kawato, M. Neural network control for a closed-loop system using feedback-error-learning. Neural Netw. 1993, 6, 933–946. [Google Scholar] [CrossRef]
- Ito, M. Cerebellar long-term depression: Characterization, signal transduction, and functional roles. Physiol. Rev. 2001, 81, 1143–1195. [Google Scholar] [CrossRef] [PubMed]
- Barmack, N.H. Central vestibular system: Vestibular nuclei and posterior cerebellum. Brain Res. Bull. 2003, 60, 511–541. [Google Scholar] [CrossRef]
- Morton, S.M.; Bastian, A.J. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J. Neurosci. 2006, 26, 9107–9116. [Google Scholar] [CrossRef] [PubMed]
- Levin, S.M.; Goldberg, M. Clinical evaluation and management of lead-exposed construction workers. Am. J. Ind. Med. 2000, 37, 23–43. [Google Scholar] [CrossRef]
- Wrobel, C.K.M. Inside the Investigation that Exposed Lead-laced Drinking Water in Canada. GLOBAL NEWS. 2019. Available online: https://globalnews.ca/news/6134683/inside-the-investigation-tainted-water-canada (accessed on 10 January 2020).
- Hohnadel, D.C.; Sunderman, F.W., Jr.; Nechay, M.W.; McNeely, M.D. Atomic absorption spectrometry of nickel, copper, zinc, and lead in sweat collected from healthy subjects during sauna bathing. Clin. Chem. 1973, 19, 1288–1292. [Google Scholar] [CrossRef]
- Kharoubi, O.; Slimani, M.; Aoues, A. Neuroprotective effect of wormwood against lead exposure. J. Emerg. Trauma Shock 2011, 4, 82–88. [Google Scholar] [CrossRef]
- Katsuyama, M.; Matsuno, K.; Yabe-Nishimura, C. Physiological roles of NOX/NADPH oxidase, the superoxide-generating enzyme. J. Clin. Biochem. Nutr. 2012, 50, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Koh, D.H.; Locke, S.J.; Chen, Y.C.; Purdue, M.P.; Friesen, M.C. Lead exposure in US worksites: A literature review and development of an occupational lead exposure database from the published literature. Am. J. Ind. Med. 2015, 58, 605–616. [Google Scholar] [CrossRef] [Green Version]
- Freire, M.A.M.; Santana, L.N.S.; Bittencourt, L.O.; Nascimento, P.C.; Fernandes, R.M.; Leao, L.K.R.; Fernandes, L.M.P.; Silva, M.C.F.; Amado, L.L.; Gomes-Leal, W.; et al. Methylmercury intoxication and cortical ischemia: Pre-clinical study of their comorbidity. Ecotoxicol. Environ. Saf. 2019, 174, 557–565. [Google Scholar] [CrossRef]
- Gu, H.; Wei, X.; Monnot, A.D.; Fontanilla, C.V.; Behl, M.; Farlow, M.R.; Zheng, W.; Du, Y. Lead exposure increases levels of β-amyloid in the brain and CSF and inhibits LRP1 expression in APP transgenic mice. Neurosci. Lett. 2011, 490, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Geist, C.R.; Mattes, B.R. Behavioral effects of postnatal lead acetate exposure in developing laboratory rats. Physiol. Psychol. 1979, 7, 399–402. [Google Scholar] [CrossRef]
- Luthman, J.; Oskarsson, A.; Olson, L.; Hoffer, B. Postnatal lead exposure affects motor skills and exploratory behavior in rats. Environ. Res. 1992, 58, 236–252. [Google Scholar] [CrossRef]
- Benammi, H.; Erazi, H.; El Hiba, O.; Vinay, L.; Bras, H.; Viemari, J.-C.; Gamrani, H. Disturbed sensorimotor and electrophysiological patterns in lead intoxicated rats during development are restored by curcumin I. PLoS ONE 2017, 12, e0172715. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.P.; Worley, P.F.; Guilarte, T.R. Lead exposure during synaptogenesis alters NMDA receptor targeting via NMDA receptor inhibition. Neurotoxicology 2011, 32, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckner, R.L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 2013, 80, 807–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorton, D.; Anderson, W.J. The effects of postnatal lead toxicity on the development of cerebellum in rats. Neurobehav. Toxicol. Teratol. 1986, 8, 51–59. [Google Scholar]
- Sidhu, P.; Nehru, B. Lead Intoxication: Histological and Oxidative Damage in Rat Cerebrum and Cerebellum. J. Trace Elem. Exp. Med. 2004, 17, 45–53. [Google Scholar] [CrossRef]
- Dumková, J.; Smutná, T.; Vrlíková, L.; Le Coustumer, P.; Večeřa, Z.; Dočekal, B.; Mikuška, P.; Čapka, L.; Fictum, P.; Hampl, A.; et al. Sub-chronic inhalation of lead oxide nanoparticles revealed their broad distribution and tissue-specific subcellular localization in target organs. Part. Fibre Toxicol. 2017, 14, 55. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.L.; Guariglia, S.R.; McGlothan, J.L.; Stansfield, K.H.; Stanton, P.K.; Guilarte, T.R. Presynaptic mechanisms of lead neurotoxicity: Effects on vesicular release, vesicle clustering and mitochondria number. PLoS ONE 2015, 10, e0127461. [Google Scholar] [CrossRef] [Green Version]
- Dabrowska-Bouta, B.; Sulkowski, G.; Bartosz, G.; Walski, M.; Rafalowska, U. Chronic lead intoxication affects the myelin membrane status in the central nervous system of adult rats. J. Mol. Neurosci. 1999, 13, 127–139. [Google Scholar] [CrossRef]
- Skoczyńska, A.; Smolik, R.; Jeleń, M. Lipid abnormalities in rats given small doses of lead. Arch. Toxicol. 1993, 67, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.S.; Hussain, T.; Chandra, S. Possible role of regional superoxide dismutase activity and lipid peroxide levels in cadmium neurotoxicity: In vivo and in vitro studies in growing rats. Life Sci. 1987, 41, 2215–2221. [Google Scholar] [CrossRef]
- Antonio, M.; Corpas, I.; Leret, M. Neurochemical changes in newborn rat’s brain after gestational cadmium and lead exposure. Toxicol. Lett. 1999, 104, 1–9. [Google Scholar] [CrossRef]
- Arundine, M.; Tymianski, M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 2003, 34, 325–337. [Google Scholar] [CrossRef]
- Hynd, M.R.; Scott, H.L.; Dodd, P.R. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2004, 45, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407–6418. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.C.; Bratton, S.B. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid. Redox Signal. 2013, 19, 546–558. [Google Scholar] [CrossRef] [Green Version]
- Bean, B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007, 8, 451–465. [Google Scholar] [CrossRef]
- Kress, G.J.; Mennerick, S. Action potential initiation and propagation: Upstream influences on neurotransmission. Neuroscience 2009, 158, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Boggs, J.M. Myelin basic protein: A multifunctional protein. Cell. Mol. life Sci. 2006, 63, 1945–1961. [Google Scholar] [CrossRef]
- Franklin, R.J.; Ffrench-Constant, C. Remyelination in the CNS: From biology to therapy. Nat. Rev. Neurosci. 2008, 9, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Duncan, I.D.; Hammang, J.P.; Goda, S.; Quarles, R.H. Myelination in the jimpy mouse in the absence of proteolipid protein. Glia 1989, 2, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Duncan, I.D.; Hammang, J.P.; Trapp, B.D. Abnormal compact myelin in the myelin-deficient rat: Absence of proteolipid protein correlates with a defect in the intraperiod line. Proc. Natl. Acad. Sci. USA 1987, 84, 6287–6291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nave, K.A.; Salzer, J.L. Axonal regulation of myelination by neuregulin 1. Curr. Opin. Neurobiol. 2006, 16, 492–500. [Google Scholar] [CrossRef]
- Kutzelnigg, A.; Faber-Rod, J.C.; Bauer, J.; Lucchinetti, C.F.; Sorensen, P.S.; Laursen, H.; Stadelmann, C.; Bruck, W.; Rauschka, H.; Schmidbauer, M.; et al. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 2007, 17, 38–44. [Google Scholar] [CrossRef]
- Siffrin, V.; Vogt, J.; Radbruch, H.; Nitsch, R.; Zipp, F. Multiple sclerosis—candidate mechanisms underlying CNS atrophy. Trends Neurosci. 2010, 33, 202–210. [Google Scholar] [CrossRef]
- Wilkins, A. Cerebellar Dysfunction in Multiple Sclerosis. Front. Neurol. 2017, 8, 312. [Google Scholar] [CrossRef] [Green Version]
- Servais, L.; Hourez, R.; Bearzatto, B.; Gall, D.; Schiffmann, S.N.; Cheron, G. Purkinje cell dysfunction and alteration of long-term synaptic plasticity in fetal alcohol syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 9858–9863. [Google Scholar] [CrossRef] [Green Version]
- Hoxha, E.; Boda, E.; Montarolo, F.; Parolisi, R.; Tempia, F. Excitability and Synaptic Alterations in the Cerebellum of APP/PS1 Mice. PLoS ONE 2012, 7, e34726. [Google Scholar] [CrossRef]
- Maycox, P.R.; Link, E.; Reetz, A.; Morris, S.A.; Jahn, R. Clathrin-coated vesicles in nervous tissue are involved primarily in synaptic vesicle recycling. J. Cell Biol. 1992, 118, 1379–1388. [Google Scholar] [CrossRef]
- Morgan, J.R.; Prasad, K.; Hao, W.; Augustine, G.J.; Lafer, E.M. A conserved clathrin assembly motif essential for synaptic vesicle endocytosis. J. Neurosci. 2000, 20, 8667–8676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, H.; Robison, G.; Hong, L.; Barrea, R.; Wei, X.; Farlow, M.R.; Pushkar, Y.N.; Du, Y.; Zheng, W. Increased beta-amyloid deposition in Tg-SWDI transgenic mouse brain following in vivo lead exposure. Toxicol. Lett. 2012, 213, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandolfo, P.; Pamplona, F.A.; Prediger, R.D.; Takahashi, R.N. Increased sensitivity of adolescent spontaneously hypertensive rats, an animal model of attention deficit hyperactivity disorder, to the locomotor stimulation induced by the cannabinoid receptor agonist WIN 55,212-2. Eur. J. Pharmacol. 2007, 563, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Šlamberová, R.; Pometlová, M.; Charousová, P. Postnatal development of rat pups is altered by prenatal methamphetamine exposure. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Green, L.C.; Ruiz de Luzuriaga, K.; Wagner, D.A.; Rand, W.; Istfan, N.; Young, V.R.; Tannenbaum, S.R. Nitrate biosynthesis in man. Proc. Natl. Acad. Sci. USA 1981, 78, 7764–7768. [Google Scholar] [CrossRef] [Green Version]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef]
- Bittencourt, L.O.; Dionizio, A.; Nascimento, P.C.; Puty, B.; Leao, L.K.R.; Luz, D.A.; Silva, M.C.F.; Amado, L.L.; Leite, A.; Buzalaf, M.R.; et al. Proteomic approach underlying the hippocampal neurodegeneration caused by low doses of methylmercury after long-term exposure in adult rats. Metallomics 2019, 11, 390–403. [Google Scholar] [CrossRef]
- Dionizio, A.; Melo, C.; Sabino-Arias, I.; Ventura, T.; Leite, A.; Souza, S.; Santos, E.; Heubel, A.; Souza, J.; Martins Perles, J.; et al. Chronic treatment with fluoride affects the jejunum: Insights from proteomics and enteric innervation analysis. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Lima Leite, A.; Gualiume Vaz Madureira Lobo, J.; Barbosa da Silva Pereira, H.A.; Silva Fernandes, M.; Martini, T.; Zucki, F.; Sumida, D.H.; Rigalli, A.; Buzalaf, M.A. Proteomic analysis of gastrocnemius muscle in rats with streptozotocin-induced diabetes and chronically exposed to fluoride. PLoS ONE 2014, 9, e106646. [Google Scholar] [CrossRef] [Green Version]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pages, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamarao-Vieira, K.; Pamplona-Santos, D.; Nascimento, P.C.; Correa, M.G.; Bittencourt, L.O.; Dos Santos, S.M.; Cartagenes, S.C.; Fernandes, L.M.P.; Monteiro, M.C.; Maia, C.S.F.; et al. Physical Exercise Attenuates Oxidative Stress and Morphofunctional Cerebellar Damages Induced by the Ethanol Binge Drinking Paradigm from Adolescence to Adulthood in Rats. Oxidative Med. Cell. Longev. 2019, 2019, 6802424. [Google Scholar] [CrossRef] [PubMed]
- Santana, L.; Bittencourt, L.O.; Nascimento, P.C.; Fernandes, R.M.; Teixeira, F.B.; Fernandes, L.M.P.; Freitas Silva, M.C.; Nogueira, L.S.; Amado, L.L.; Crespo-Lopez, M.E.; et al. Low doses of methylmercury exposure during adulthood in rats display oxidative stress, neurodegeneration in the motor cortex and lead to impairment of motor skills. J. Trace Elem. Med. Biol. 2019, 51, 19–27. [Google Scholar] [CrossRef] [PubMed]












| Accesion ID a | Protein Description | Score | Fold Change |
|---|---|---|---|
| P60203 | Myelin proteolipid protein | 794.51 | −0.92 |
| P19511 | ATP synthase F(0) complex subunit B1, mitochondrial | 212.89 | −0.44 |
| P11442 | Clathrin heavy chain 1 | 174.43 | 1.06 |
| P06761 | Endoplasmic reticulum chaperone BiP | 453.96 | 1.11 |
| F1LP05 | ATP synthase subunit alpha | 2920.7 | 1.12 |
| P15999 | ATP synthase subunit alpha, mitochondrial | 3053 | 1.12 |
| P31399 | ATP synthase subunit d, mitochondrial | 665.55 | 1.12 |
| P07895 | Superoxide dismutase [Mn], mitochondrial | 333.18 | 1.12 |
| Q9R063 | Peroxiredoxin-5, mitochondrial | 596.72 | 1.15 |
| P18418 | Calreticulin | 135.67 | 1.16 |
| P35434 | ATP synthase subunit delta, mitochondrial | 314.76 | 1.17 |
| P10719 | ATP synthase subunit beta, mitochondrial | 8483 | 1.20 |
| Q9QUL6 | Vesicle-fusing ATPase | 130.82 | 1.25 |
| G3V9G3 | Calcium/calmodulin-dependent protein kinase II, beta, isoform CRA_a | 317.64 | 1.52 |
| G3V9G3 | Calcium/calmodulin-dependent protein kinase II, beta, isoform CRA_a | 317.64 | 1.52 |
| P11275 | Calcium/calmodulin-dependent protein kinase type II subunit alpha | 164.49 | 1.65 |
| Q9JHZ4 | GRIP1-associated protein 1 | 30,243 | Pb |
| +631 proteins with different status of regulation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leão, L.K.R.; Bittencourt, L.O.; Oliveira, A.C.; Nascimento, P.C.; Miranda, G.H.N.; Ferreira, R.O.; Nabiça, M.; Dantas, K.; Dionizio, A.; Cartágenes, S.; et al. Long-Term Lead Exposure Since Adolescence Causes Proteomic and Morphological Alterations in the Cerebellum Associated with Motor Deficits in Adult Rats. Int. J. Mol. Sci. 2020, 21, 3571. https://doi.org/10.3390/ijms21103571
Leão LKR, Bittencourt LO, Oliveira AC, Nascimento PC, Miranda GHN, Ferreira RO, Nabiça M, Dantas K, Dionizio A, Cartágenes S, et al. Long-Term Lead Exposure Since Adolescence Causes Proteomic and Morphological Alterations in the Cerebellum Associated with Motor Deficits in Adult Rats. International Journal of Molecular Sciences. 2020; 21(10):3571. https://doi.org/10.3390/ijms21103571
Chicago/Turabian StyleLeão, Luana Ketlen Reis, Leonardo Oliveira Bittencourt, Ana Carolina Oliveira, Priscila Cunha Nascimento, Giza Hellen Nonato Miranda, Railson Oliveira Ferreira, Mariane Nabiça, Kelly Dantas, Aline Dionizio, Sabrina Cartágenes, and et al. 2020. "Long-Term Lead Exposure Since Adolescence Causes Proteomic and Morphological Alterations in the Cerebellum Associated with Motor Deficits in Adult Rats" International Journal of Molecular Sciences 21, no. 10: 3571. https://doi.org/10.3390/ijms21103571