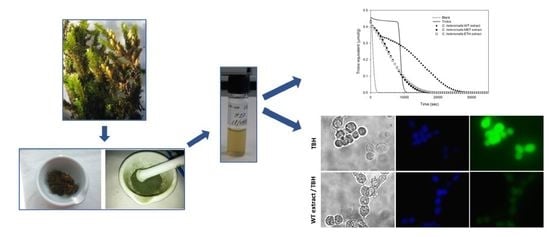
Water Extract of Cryphaea heteromalla (Hedw.) D. Mohr Bryophyte as a Natural Powerful Source of Biologically Active Compounds
Abstract
:1. Introduction
2. Results
2.1. Total Biophenols and the Antioxidant Ability of WT, MET, and ETH Cryphaea heteromalla Extracts
2.2. Chemical Characterization of the Cryphaea heteromalla WT Extract
2.3. WT Extract Protective Effect against Oxidative Stress
3. Discussions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Cryphaea heteromalla Water Extracts
4.3. Determination of Total Biophenol Content
4.4. Oxygen Radical Absorbance Capacity (ORAC) Assay
4.5. HPLC-ESI-TOF-MS Chemical Analysis
4.6. Cell Culture
4.7. Cell Viability Assay
4.8. Protection Analysis against Oxidative Stress
4.9. Evaluation of the Cell ROS Content
4.10. ROS Detection by Fluorescence Microscopy
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shaw, A.J.; Szövényi, P.P.; Shaw, B. Bryophyte diversity and evolution: Windows into the early evolution of land plants. Am. J. Bot. 2011, 98, 352–369. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Ludwiczuk, A. Chemical costituents of bryophytes: Structures and biological activity. J. Nat. Prod. 2018, 81, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Chandra, D.; Barh, A.; Pankaj; Pandey, R.K.; Sharma, I.P. Bryophytes: Hoard of remedies, and ethno-medicinal review. J. Tradit. Complement. Med. 2017, 7, 94–98. [Google Scholar]
- Li, S. Compendium of Materia Medica (Benca Gangmu); Foreing Languages Press: Bejing, China, 2004. [Google Scholar]
- Tonguc-Yayintas, O.; Irkin, L.C. Bryophytes as hidden treasure. J. Sci. Perspect. 2018, 2, 71–83. [Google Scholar] [CrossRef]
- Slack, N.G. The ecological value of bryophytes as indicators of climate change. In Bryophyte Ecology and Climate Change; Tuba, Z., Slack, N.G., Stark, L.R., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 3–11. [Google Scholar]
- Basile, A.; Giordano, S.; Cafiero, G.; Spagnuolo, V.; Castaldo, C.R. Tissue and cell localization of experimentally supplied Pb in F. hygrometrica (Hedw) using X-ray SEM and TEM microanalysis. J. Bryol. 1994, 18, 69–81. [Google Scholar] [CrossRef]
- Basile, A.; Cogoni, A.E.; Bassi, P.; Fabrizi, E.; Sorbo, S.; Giordano, S.; Castaldo Cobianchi, R. Accumulation of Pb and Zn in gametophytes and sporophytes of the moss Funaria hygrometrica (Funariales). Ann. Bot. 2001, 87, 537–543. [Google Scholar] [CrossRef]
- Vuotto, M.L.; De Sole, P.; Castaldo Cobianchi, R.; Sorbo, S.; Sepe, J.; Faiella, M.R.; Miranda, R.; Ricciardi, L.; Spatuzzi, D.; De Prisco, R.; et al. Antioxidant activity in extracts from Pleurochae tesquarrosa (Bryophyta) stressed by heavy metals, heat shock and salinity. In Bioluminescence and Chemiluminescence, Progress and Current Applications; Stanley, P.E., Kricka, L.J., Eds.; World Scientific: Singapore, 2002; pp. 301–304. [Google Scholar]
- Dey, A.; De, J.N. Antioxidative potential of bryophytes: Stress tolerance and commercial perspectives. A review. Pharmacologia 2011, 3, 151–159. [Google Scholar] [CrossRef]
- Singh, M.; Singh, S.; Nath, V.; Sahu, V.; Singh Rawat, A.K. Antibacterial activity of some bryophytes used traditionally for the treatment of burn infections. Pharm. Biol. 2011, 49, 526–530. [Google Scholar] [CrossRef]
- Dierßen, K. Distribution, ecological amplitude and phytosociological characterization of European bryophytes. Bryophyt. Bibl. 2001, 56, 1–289. [Google Scholar]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.M.; Pessarakli, M. Reactive oxygen species, oxidative damage and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Giacomazza, D.; D’Andrea, D.; Provenzano, A.; Picone, P.; Provenzano, F.; Guarrasi, V.; Raimondo, M.; San Biagio, P.L.; Passantino, R.; Mangione, M.R.; et al. The precious content of the olive mill wastewater: The protective effect of the antioxidant fraction in cell cultures. Cyta-J. Food 2018, 16, 658–666. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Drabinska, N.; Ciska, E.; Szmatowicz, B.; Krupa-Kozak, U. Broccoli by-products improve the nutraceutical potential of gluten-free mini sponge cakes. Food Chem. 2018, 267, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Adebiyi, A.O.; Oyedeji, A.A.; Chikwendu, E.E.; Fatoke, O.A. Phytochemical screening of two tropical moss plants: Thidium gratum P. Beauv and Barbula indica Brid grown in southwestern ecological zone of Nigeria. Am. J. Anal. Chem. 2012, 3, 836–839. [Google Scholar] [CrossRef]
- Karim, F.A.; Suleiman, M.; Rahmat, A.; Bakar, M.F.A. Phytochemicals, antioxidant and antiproliferative properties of five moss species from Sabha, Malaysia. Int. J. Pharm. Pharm. Sci. 2014, 6, 292–297. [Google Scholar]
- Moukette, B.M.; Pieme, C.A.; Njimou, J.R.; Biapa, C.P.N.; Bravi, M.; Ngogang, J.Y. In vitro antioxidant properties, free radicals scavenging activities of extracts and polyphenol composition of a non-timber forest product used as spice: Monodora myristica. Biol. Res. 2015, 48, 15. [Google Scholar] [CrossRef]
- Presti, G.; Guarrasi, V.; Gulotta, E.; Provenzano, F.; Provenzano, A.; Giuliano, S.; Monfeda, M.; Mangione, M.R.; Passantino, R.; San Biagio, P.L.; et al. Bioactive compounds from extra virgin olive oils: Correlation between phenolic content and oxidative stress cell protection. Biophys. Chem. 2017, 230, 109–116. [Google Scholar] [CrossRef]
- Gurau, F.; Baldoni, S.; Prattichizzo, F.; Espinosa, E.; Amenta, F.; Procopio, A.D.; Albertini, M.C.; Bonafè, M.; Olivieri, F. Anti-senescence compounds: A potential nutraceutical approach to healthy Aging. Ageing Res. Rev. 2018, 46, 14–31. [Google Scholar] [CrossRef]
- Mao, X.; Gu, C.; Chen, D.; Yu, B.; He, J. Oxidative stress-induced diseases and tea polyphenols. Oncotarget 2017, 8, 81649–81661. [Google Scholar] [CrossRef] [Green Version]
- Zanzer, Y.C.; Plaza, M.; Dougkas, A.; Turner, C.; Ostman, E. Black pepper-based beverage induced appetite-suppressing effects without altering postprandial glycaemia, gut and thyroid hormones or gastrointestinal well-being: A randomized crossover study in healthy subjects. Food Funct. 2018, 9, 2774–2786. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Ouyang, H.; Lu, Y.; Qiu, Y.; Feng, Y.; Jiang, H.; Zhou, X.; Yang, S. A novel dereplication strategy for the identification of two new trace compounds in the extract of Gastrodia elata using UHPLC/Q-TOF-MS/MS. J. Chromatogr. B 2015, 988, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.T.; Lu, Y.F.; Stephen Inbaraj, B.; Chen, B.H. Determination of phenolic acids and flavonoids in Rhinacanthus nasutus (L.) kurz by high performance-liquid-chromatography with photodiode-array detection and tandem mass spectrometry. J. Funct. Food 2015, 12, 498–508. [Google Scholar] [CrossRef]
- Lai, C.-J.-S.; Zha, L.; Liu, D.-H.; Kang, L.; Ma, X.; Zhan, Z.-L.; Nan, T.-G.; Yang, J.; Li, F.; Yuan, Y.; et al. Global profiling and rapid matching of natural products using diagnostic product ion network and in silico analogue database: Gastrodia elata as a case study. J. Chromatogr. A 2016, 1456, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Quifer-Rada, P.; Vallverdu-Queralt, A.; MMartinez-Huelamo, M.; Chiva-Blanch, G.; Jauregui, O.; Estruch, R.; Lamuela-Raventos, R. A comprehensive characterisation of beer polyphenols by high resolution mass spectrometry (LC–ESI-LTQ-Orbitrap-MS). Food Chem. 2015, 169, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Strnad, M. Jasmonates: News on occurrence, biosynthesis, metabolism and action of an ancient group of signaling compounds. Int. J. Mol. Sci. 2018, 19, 2539. [Google Scholar] [CrossRef] [PubMed]
- Eng, F.; Haroth, S.; Feussner, K.; Meldau, D.; Rekhter, D.; Ischebeck, T.; Brodhum, F.; Feussner, I. Optimized jasmonic acid production by Lasiodiplodia theobromae reveals formation of valuable plant secondary metabolites. PLoS ONE 2016, 11, e0167627. [Google Scholar] [CrossRef]
- Flokova, K.; Tarkowska, D.; Miersch, O.; Strnad, M.; Wasternack, C.; Novak, O. UHPLC–MS/MS based target profiling of stress-induced phytohormones. Phytochemistry 2014, 105, 147–157. [Google Scholar] [CrossRef]
- Boccard, J.; Grata, E.; Thiocone, A.; Gauvrit, J.-Y.; Lanteri, P.; Carrupt, P.-A.; Wolfender, J.-L.; Rudaz, S. Multivariate data analysis of rapid LC-TOF/MS experiments from Arabidopsis thaliana stressed by wounding. Chemom. Intell. Lab. Syst. 2007, 86, 189–197. [Google Scholar] [CrossRef]
- Meulebroek, L.V.; Bussche, J.V.; Steppe, K.; Vanhaecke, L. Ultra-high performance liquid chromatography coupled to high resolution Orbitrap mass spectrometry for metabolomic profiling of the endogenous phytohormonal status of the tomato plant. J. Chromatogr. A 2012, 1260, 67–80. [Google Scholar] [CrossRef]
- Thiocone, A.; Farmer, E.E.; Wolfender, J.-L. Screening for wound-induced oxylipins in Arabidopsis thaliana by differential HPLC-APCI/MS profiling of crude leaf extracts and subsequent characterization by capillary-scale NMR. Phytochem. Anal. 2008, 19, 198–205. [Google Scholar] [CrossRef]
- Zhai, Q.; Yan, C.; Li, L.; Xie, D.; Li, C. Jasmonates. In Hormone Metabolism and Signaling in Plants, 1st ed.; Li, J., Li, C., Smith, S., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 243–271. [Google Scholar]
- Cohen, S.; Flescher, E. Methyl jasmonate: A plant stress hormone as an anti-cancer drug. Phytochemistry 2009, 70, 1600–1609. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Di Carlo, M.; Giacomazza, D.; Picone, P.; Nuzzo, D.; San Biagio, P.L. Are oxidative stress and mitochondrial dysfunction the key players in the neurodegenerative diseases? Free Radic. Res. 2012, 46, 1327–1338. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.R. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef]
- Misra, M.K.; Sarwat, M.; Bhakuni, P.; Tuteja, R.; Tuteja, N. Oxidative stress and ischemic myocardial syndromes. Med. Sci. Monit. 2009, 15, 209–219. [Google Scholar]
- Noda, N.; Wakasugi, H. Cancer and oxidative stress. J. Jpn. Med. Assoc. 2001, 24, 1571–1574. [Google Scholar]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Namiki, M. Antioxidants/antimutagens in food. Food Sci. Nutr. 1990, 29, 273–300. [Google Scholar] [CrossRef]
- Modi, K.K.; Roy, A.; Brahmachari, S.; Rangasamy, S.B.; Pahan, K. Cinnamon and its metabolite sodium benzoate attenuate the activation of p21rac and protect memory and learning in an animal model of Alzheimer’s disease. PLoS ONE 2015, 10, e0130398. [Google Scholar] [CrossRef]
- Reis, B.; Martins, M.; Barreto, B.; Milhazes, N. Structure-property-activity relationship of phenolic acids and derivatives. Protocatechuic acid alkyl esters. J. Agric. Food. Chem. 2010, 58, 6986–6993. [Google Scholar] [CrossRef]
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic acid antioxidants: An electrochemical overview. BioMed Res. Int. 2013, 2013, 251754. [Google Scholar] [CrossRef]
- Touaibia, M.; Jean-Francois, J.; Doiron, J. Caffeic Acid, a versatile pharmacophore: An overview. Mini Rev. Med. Chem. 2011, 11, 695–713. [Google Scholar] [CrossRef]
- Taofiq, O.; Gonzales-Paramas, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R. Hydroxycinnamic acids and their derivatives: Cosmeceutical significance, challenges and future perspectives, a review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Karthikesan, K.; Pari, L. Beneficial effect of caffeic acid on alcohol-induced alterations in lipid peroxidation and antioxidants defense in rats. Toxicol. Mech. Methods 2007, 17, 527–534. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.J.; Jiang, F.; Yang, Y.; Wang, X.X.; Zhang, Z.; Li, Z.; Li, L. Caffeic acid improves cell viability and protects against DNA damage: Involvement of reactive oxygen species and extracellular signal-regulated kinase. Braz. J. Med. Biol. Res. 2015, 48, 502–508. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Pyo, M.C.; Nam, M.-H.; Lee, K.-W. Erk/Nrf2 pathway activation by caffeic acid in HepG2 cells alleviates its hepatocellular damage caused by t-butylhydroperoxide-induced oxidative stress. BMC Complement. Altern. Med. 2019, 19, 139. [Google Scholar] [CrossRef]
- Bouzaiene, N.N.; Kilani-Jaziri, S.; Kovacic, H.; Chekir-Ghedira, L.; Ghedira, K.; Luis, J. The effects of caffeic, coumaric and ferulic acids on proliferation, superoxide production, adhesion and migration of human tumor cells in vitro. Eur. J. Pharmacol. 2015, 766, 99–105. [Google Scholar] [CrossRef]
- Gum, R.; Wang, H.; Lengyel, E.; Juarez, J.; Boyd, D. Regulation of 92 kDa type IV collagenase expression by the jun amino terminal kinase and the extracellular signal-regulated kinase-dependent signaling cascades. Oncogene 1997, 14, 1481–1493. [Google Scholar] [CrossRef]
- Takahashi, T.; Miyazawa, M. Tyrosinase inhibitory activities of cinnamic acid analogues. Pharmazie 2010, 65, 913–918. [Google Scholar]
- Maeda, K.; Fukuda, M. In vitro effectiveness of several whitening cosmetic components in human melanocytes. J. Soc. Cosmet. Chem. 1991, 42, 361–368. [Google Scholar]
- Lafay, S.; Gueux, E.; Rayssiguier, Y.; Mazur, A.; Rémésy, C.; Scalbert, A. Caffeic acid inhibits oxidative stress and reduces hypercholesterolemia induced by iron overloads in rats. Int. J. Vitam. Nutr. Res. 2005, 75, 119–125. [Google Scholar] [CrossRef]
- Zang, L.-Y.; Cosma, G.; Gardner, H.; Shi, X.; Castranova, V.; Vallyathan, V. Effect of antioxidant protection by p-coumaric acid on low-density lipoprotein cholesterol oxidation. Am. J. Physiol. Cell Physiol. 2000, 279, C954–C960. [Google Scholar] [CrossRef]
- Ursini, F.; Leporini, C.; Bene, F.; D’Angelo, S.; Mauro, D.; Russo, E.; De Sarro, G.; Olivieri, I.; Pitzalis, C.; Lewis, M.; et al. Anti-TNF-alpha agents and endothelial function in rheumatoid arthritis: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 5346. [Google Scholar] [CrossRef]
- Peng, J.; Zheng, T.-T.; Liang, Y.; Duan, L.-F.; Zhang, Y.-D.; Wang, L.-J.; He, G.-M.; Xiao, H.T. p-coumaric acid protects human lens epithelial cells against oxidative stress-induced apoptosis by MAPK signaling. Oxidative Med. Cell. Longev. 2018, 2018, 8549052. [Google Scholar] [CrossRef]
- Eduviere, A.T.; Umukoro, S.; Aderibigbe, A.O.; Ajayi, A.M.; Adewole, F.A. Methyl jasmonate enhances memory performance through inhibition of oxidative stress and acetylcholinesterase activity in mice. Life Sci. 2015, 132, 20–26. [Google Scholar] [CrossRef]
- Taki-Nakano, N.; Ohzrki, H.; Kotera, J.; Ohta, H. Cytoprotective effects of 12-oxo phytodienoic acid, a plant-derived oxylipin jasmonate, on oxidative stress-induced toxicity in human neuroblastoma SH-SY5Y cells. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 3413–3422. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, J.A.; Klegeris, A. Modulation of microglial functions by methyl jasmonate. Neural Regen. Res. 2018, 13, 1290–1293. [Google Scholar]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef]
- Hrncirik, K.; Frische, S. Comparability and reliability of different techniques for the determination of phenolic compounds in virgin olive oil. Eur. J. Lipid Sci. Technol. 2004, 106, 540–549. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. Postprandial increases in serum antioxidant capacity in older women. J. Appl. Physiol. 2000, 89, 877–883. [Google Scholar] [CrossRef]
- Ninfali, P.; Bacchiocca, M.; Biagiotti, E.; Servili, M.; Montedoro, G. Validation of the Oxygen Radical Absorbance Capacity (ORAC) parameter as a new index of quality and stability of virgin olive oil. JAOCS 2002, 79, 977–982. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]







| Extraction Time | WT | MET | ETH |
|---|---|---|---|
| 1 | 2.36 ± 0.04 | 0.87 ± 0.04 | 0.66 ± 0.04 |
| 2 | 2.45 ± 0.04 | 1.00 ± 0.01 | 0.72 ± 0.03 |
| 3 | 3.34 ± 0.04 | 1.28 ± 0.02 | 1.22 ± 0.07 |
| 4 | 3.36 ± 0.07 | 1.26 ± 0.03 | 1.18 ± 0.04 |
| Extraction Time | WT | MET | ETH |
|---|---|---|---|
| 1 | 47.33 ± 0.3 | 21.25 ± 0.5 | 22.03 ± 0.5 |
| 2 | 50.51 ± 1.0 | 23.42 ± 0.3 | 24.32 ± 0.5 |
| 3 | 52.53 ± 1.0 | 25.36 ± 1.1 | 26.67 ± 0.5 |
| Peak No. | RT (min) | m/z Experimental | Molecular Formula (M–H) | m/z Calculated | Error (ppm) | mSigma | Proposed Compound |
|---|---|---|---|---|---|---|---|
| 1 | 2.64 | 201.0261 | C4H9O 9 | 201.0252 | –4.4 | 149.5 | Not identified |
| 2 | 3.9 | 385.1359 | C14H25O12 | 385.1351 | –1.9 | 17.9 | Sugar derivative |
| 3 | 6.22 | 251.0814 | C9H15O8 | 251.0772 | –16.5 | 4.4 | Not identified |
| 4 | 8.46 | 447.1226 | C18H23O13 | 447.1144 | –18.4 | 10.4 | Di-hydroxybenzoic acid hexoside–pentoside |
| 5 | 10.03 | 473.1386 | C20H25O13 | 473.1301 | –18.1 | 5 | Caffeic acid hexoside-pentoside or isomer |
| 6 | 10.84 | 473.1406 | C20H25O13 | 473.1301 | 18.3 | 7.5 | Caffeic acid hexoside pentoside or isomer |
| 7 | 19.82 | 327.2227 | C18H31O5 | 327.2177 | –15.3 | 13.9 | Dihydro-p-coumaric acid hexoside |
| 8 | 21.02 | 329.2380 | C18H33O5 | 329.2333 | –14.3 | 5.6 | Hydrated-dihydro-p-coumaric acid hexoside |
| 9 | 23.01 | 239.1687 | C14H23O3 | 239.1653 | –14.5 | 10.6 | Hydrated-3-oxo-2-(pent-2-enyl)-cyclopentane-1-butanoic acid (hydrated OPC-4) |
| 10 | 24.7 | 237.1523 | C14H21O3 | 237.1496 | –11.5 | 10 | 3-Oxo-2-(pent-2-enyl)-cyclopentane-1-butanoic acid (OPC-4) |
| 11 | 25.4 | 263.1684 | C16H23O3 | 263.1653 | –11.9 | 22 | Dinor-oxo-phytodienoic acid |
| 12 | 27.34 | 315.2569 | C18H35O4 | 315.2541 | –8.9 | 14.1 | Not identified |
| 13 | 28.9 | 265.1836 | C16H25O3 | 265.1809 | –10.2 | 4.9 | 3-Oxo-2-(2-(Z)-pentenyl) cyclopentane-1-hexanoic acid OPC 6 |
| 14 | 29.63 | 291.1985 | C18H27O3 | 291.1966 | –6.8 | 14.6 | cis-12-Oxo-phytodienoic acid |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Provenzano, F.; Sánchez, J.L.; Rao, E.; Santonocito, R.; Ditta, L.A.; Borrás Linares, I.; Passantino, R.; Campisi, P.; Dia, M.G.; Costa, M.A.; et al. Water Extract of Cryphaea heteromalla (Hedw.) D. Mohr Bryophyte as a Natural Powerful Source of Biologically Active Compounds. Int. J. Mol. Sci. 2019, 20, 5560. https://doi.org/10.3390/ijms20225560
Provenzano F, Sánchez JL, Rao E, Santonocito R, Ditta LA, Borrás Linares I, Passantino R, Campisi P, Dia MG, Costa MA, et al. Water Extract of Cryphaea heteromalla (Hedw.) D. Mohr Bryophyte as a Natural Powerful Source of Biologically Active Compounds. International Journal of Molecular Sciences. 2019; 20(22):5560. https://doi.org/10.3390/ijms20225560
Chicago/Turabian StyleProvenzano, Fiorenza, Jesús Lozano Sánchez, Estella Rao, Radha Santonocito, Lorena Anna Ditta, Isabel Borrás Linares, Rosa Passantino, Patrizia Campisi, Maria Giovanna Dia, Maria Assunta Costa, and et al. 2019. "Water Extract of Cryphaea heteromalla (Hedw.) D. Mohr Bryophyte as a Natural Powerful Source of Biologically Active Compounds" International Journal of Molecular Sciences 20, no. 22: 5560. https://doi.org/10.3390/ijms20225560