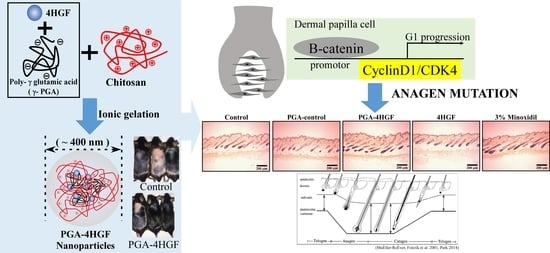
Hair Growth Promoting Effect of 4HGF Encapsulated with PGA Nanoparticles (PGA-4HGF) by β-Catenin Activation and Its Related Cell Cycle Molecules
Abstract
:1. Introduction
2. Results
2.1. Preparation, Characterization and Release Amount of PGA-4HGF
2.2. Effects of PGA-4HGF on Primary Dermal Papilla Cells Proliferation and HaCaT Cells
2.3. Hair-Growth Effects of PGA-4HGF in Telogenic C57BL/6N Mice
2.4. Anagen Phase Induction in PGA-4HGF Treated C57BL/6N Mice
2.5. Effects of PGA-4HGF on Wnt/β-Catenin Signaling and Its Related Cell Cycle Molecules
2.6. Identification of Keratin Proteins in the Dorsal Skins of PGA-4HGF-Treated Mice Using Two-Dimensional Electrophoresis (2-DE) and Peptide Mass Fingerprinting (PMF)
3. Discussion
4. Materials and Methods
4.1. Preparation and Characterization of the 4HGF Loaded Nanoparticles (PGA-4HGF)
4.2. HGF Release from the Nanoparticles (PGA-4HGF)
4.3. Cell Culture and Proliferation of Primary DPCs Using Real Time Microscopy
4.4. Cell Proliferation of Human HaCaT Cells
4.5. Anagen Phase Induction in C57BL/6N Mice
4.6. Toxicity Test of PGA-4HGF
4.7. Hair Follicle Counting and Hair Length Determination
4.8. Histological Preparation and Hematoxylin-Eosin Staining
4.9. Immunohistochemistry
4.10. Western Blot Analysis
4.11. Sample Preparation for Keratin Protein Analysis
4.12. Protein Identification by Two-Dimensional Electrophoresis (2-DE) and Peptide Mass Fingerprinting (PMF)
4.13. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daniells, S.; Hardy, G. Hair loss in long-term or home parenteral nutrition: Are micronutrient deficiencies to blame? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-I.; Kim, M.-K.; Lee, J.-H.; Jeon, Y.-J.; Hwang, E.-K.; Koh, Y.-S.; Hyun, J.-W.; Kwon, S.-Y.; Yoo, E.-S.; Kang, H.-K. Undariopsis peterseniana promotes hair growth by the activation of Wnt/β-catenin and ERK pathways. Mar. Drugs 2017, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-I.; Yoon, H.-S.; Kim, S.; Park, J.; Hyun, Y.; Ko, A.; Ahn, Y.-S.; Koh, Y.; Hyun, J.; Yoo, E.-S. Mackerel-Derived Fermented Fish Oil Promotes Hair Growth by Anagen-Stimulating Pathways. Int. J. Mol. Sci. 2018, 19, 2770. [Google Scholar] [CrossRef] [PubMed]
- Cotsarelis, G.; Millar, S.E. Towards a molecular understanding of hair loss and its treatment. Trends Mol. Med. 2001, 7, 293–301. [Google Scholar] [CrossRef]
- Park, H.-J. CARI ONE induces anagen phase of telogenic hair follicles through regulation of β-catenin, stimulation of dermal papilla cell proliferation, and melanogenesis. J. Diet. Suppl. 2014, 11, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Georgala, S.; Befon, A.; Maniatopoulou, E.; Georgala, C. Topical use of minoxidil in children and systemic side effects. Dermatology 2006, 214, 101. [Google Scholar] [CrossRef]
- Park, H.-J.; Zhang, N.; Park, D.K. Topical application of Polygonum multiflorum extract induces hair growth of resting hair follicles through upregulating Shh and β-catenin expression in C57BL/6 mice. J. Ethnopharmacol. 2011, 135, 369–375. [Google Scholar] [CrossRef]
- Li, Y.; Han, M.; Lin, P.; He, Y.; Yu, J.; Zhao, R. Hair growth promotion activity and its mechanism of Polygonum multiflorum. Evid. -Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Andersen, F.A. Final report on the safety assessment of Cocos nucifera (coconut) oil and related ingredients. Int. J. Toxicol. 2011, 30, 5S–16S. [Google Scholar] [CrossRef]
- Kappally, S.; Shirwaikar, A.; Shirwaikar, A. Coconut oil–a review of potential applications. Hygeia J. Drugs Med. 2015, 7, 34–41. [Google Scholar]
- Park, H.-J.; Han, E.S.; Park, D.K.; Lee, C.; Lee, K.W. An extract of Phellinus linteus grown on germinated brown rice inhibits inflammation markers in RAW264. 7 macrophages by suppressing inflammatory cytokines, chemokines, and mediators and up-regulating antioxidant activity. J. Med. Food 2010, 13, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Han, E.S.; Oh, J.Y.; Park, H.-J. Cordyceps militaris extract suppresses dextran sodium sulfate-induced acute colitis in mice and production of inflammatory mediators from macrophages and mast cells. J. Ethnopharmacol. 2011, 134, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Park, D.K.; Park, H.-J. Ethanol extract of Cordyceps militaris grown on germinated soybeans attenuates dextran-sodium-sulfate-(DSS-) induced colitis by suppressing the expression of matrix metalloproteinases and inflammatory mediators. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Turkoglu, M.; Pekmezci, E.; Kilic, S.; Dundar, C.; Sevinc, H. Effect of Ficus carica leaf extract on the gene expression of selected factors in HaCaT cells. J. Cosmet. Dermatol. 2017, 16, e54–e58. [Google Scholar] [CrossRef] [PubMed]
- Keresztessy, Z.; Bodnár, M.; Ber, E.; Hajdu, I.; Zhang, M.; Hartmann, J.F.; Minko, T.; Borbély, J. Self-assembling chitosan/poly-γ-glutamic acid nanoparticles for targeted drug delivery. Colloid Polym. Sci. 2009, 287, 759–765. [Google Scholar] [CrossRef]
- Hajdu, I.; Bodnár, M.; Filipcsei, G.; Hartmann, J.F.; Daróczi, L.; Zrínyi, M.; Borbély, J. Nanoparticles prepared by self-assembly of chitosan and poly-γ-glutamic acid. Colloid Polym. Sci. 2008, 286, 343–350. [Google Scholar] [CrossRef]
- Patzelt, A.; Richter, H.; Knorr, F.; Schäfer, U.; Lehr, C.-M.; Dähne, L.; Sterry, W.; Lademann, J. Selective follicular targeting by modification of the particle sizes. J. Control. Release 2011, 150, 45–48. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, J.H.; Hong, Y.S. Development of chitosan/poly-γ-glutamic acid/pluronic/curcumin nanoparticles in chitosan dressings for wound regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 81–90. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Tsai, S.-P.; Wang, D.-M.; Chang, Y.-N.; Hsieh, H.-J. Preparation of γ-PGA/chitosan composite tissue engineering matrices. Biomaterials 2005, 26, 5617–5623. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Li, Y.; Lv, M.; Li, P.; Xu, H.; Wang, L. Preparation and characterization of novel curdlan/chitosan blending membranes for antibacterial applications. Carbohydr. Polym. 2011, 84, 952–959. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Liu, W.; Lu, C.; Wang, L. Chitosan microparticles ionically cross-linked with poly (γ-glutamic acid) as antimicrobial peptides and nitric oxide delivery systems. Biochem. Eng. J. 2015, 95, 78–85. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.; Liu, T.; Prow, T. Nano-and Microtechnology in Skin Delivery of Vaccines. In Micro and Nanotechnology in Vaccine Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 327–341. [Google Scholar]
- Miljković, S.; Tomić, M.; Hut, I.; Pelemis, S. Nanomaterials for Skin Care. In Commercialization of Nanotechnologies—A Case Study Approach; Springer: Berlin/Heidelberg, Germany, 2018; pp. 205–226. [Google Scholar]
- Choi, J.-C.; Uyama, H.; Lee, C.-H.; Sung, M.-H. In Vivo Hair Growth Promotion Effects of Ultra-High Molecular Weight Poly-γ-Glutamic Acid from Bacillus subtilis (Chungkookjang). J. Microbiol. Biotechnol. 2015, 25, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Osada, A.; Iwabuchi, T.; Kishimoto, J.; Hamazaki, T.S.; Okochi, H. Long-term culture of mouse vibrissal dermal papilla cells and de novo hair follicle induction. Tissue Eng. 2007, 13, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.-Y.; Shin, Y.-H.; Yoon, H.-H.; Seo, Y.-K.; Park, J.-K. Hair follicular cell/organ culture in tissue engineering and regenerative medicine. Biochem. Eng. J. 2010, 48, 323–331. [Google Scholar] [CrossRef]
- Kiratipaiboon, C.; Tengamnuay, P.; Chanvorachote, P. Glycyrrhizic acid attenuates stem cell-like phenotypes of human dermal papilla cells. Phytomedicine 2015, 22, 1269–1278. [Google Scholar] [CrossRef]
- Ma, L.; Liu, J.; Wu, T.; Plikus, M.; Jiang, T.-X.; Bi, Q.; Liu, Y.-H.; Müller-Röver, S.; Peters, H.; Sundberg, J.P. Cyclic alopecia9 in Msx2 mutants: Defects in hair cycling and hair shaft differentiation. Development 2003, 130, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; Singh, A.T.; Mukherjee, A.; Bhat, B.; Ramesh, B.; Burman, A.C. Eclipta alba extract with potential for hair growth promoting activity. J. Ethnopharmacol. 2009, 124, 450–456. [Google Scholar] [CrossRef]
- Ito, M.; Yang, Z.; Andl, T.; Cui, C.; Kim, N.; Millar, S.E.; Cotsarelis, G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 2007, 447, 316. [Google Scholar] [CrossRef]
- Kwack, M.H.; Kang, B.M.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Minoxidil activates β-catenin pathway in human dermal papilla cells: A possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef]
- Leirós, G.J.; Ceruti, J.M.; Castellanos, M.L.; Kusinsky, A.G.; Balañá, M.E. Androgens modify Wnt agonists/antagonists expression balance in dermal papilla cells preventing hair follicle stem cell differentiation in androgenetic alopecia. Mol. Cell. Endocrinol. 2017, 439, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, S.R.; Mallinger, A.; Workman, P.; Clarke, P.A. Inhibitors of cyclin-dependent kinases as cancer therapeutics. Pharmacol. Ther. 2017, 173, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Swanton, C. Cell-cycle targeted therapies. Lancet Oncol. 2004, 5, 27–36. [Google Scholar] [CrossRef]
- Nanashima, N.; Ito, K.; Ishikawa, T.; Nakano, M.; Nakamura, T. Damage of hair follicle stem cells and alteration of keratin expression in external radiation-induced acute alopecia. Int. J. Mol. Med. 2012, 30, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Gharahdaghi, F.; Mische, S.M. Routine identification of proteins from sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels or polyvinyl difluoride membranes using matrix assisted laser desorption/ionization-time of flight-mass spectrometry (MALDI-TOF-MS). Electrophoresis 1998, 19, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Richter, H.; Teichmann, A.; Otberg, N.; Blume-Peytavi, U.; Luengo, J.; Weiss, B.; Schaefer, U.F.; Lehr, C.-M.; Wepf, R. Nanoparticles–an efficient carrier for drug delivery into the hair follicles. Eur. J. Pharm. Biopharm. 2007, 66, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-L.; Aljuffali, I.A.; Li, Y.-C.; Fang, J.-Y. Delivery and targeting of nanoparticles into hair follicles. Ther. Deliv. 2014, 5, 991–1006. [Google Scholar] [CrossRef]
- Pereira, M.N.; Ushirobira, C.Y.; Cunha-Filho, M.S.; Gelfuso, G.M.; Gratieri, T. Nanotechnology advances for hair loss. Ther. Deliv. 2018, 9, 593–603. [Google Scholar] [CrossRef]
- Spellman, M.C.; Ramirez, J. A comparison of patterns of deposition of two formulations of benzoyl peroxide on the skin and in the follicular ostia as visualized by scanning electron microscopy. In Proceedings of the Poster Presented at the 65th Annual Meeting of the American Academy of Dermatology, Washington, DC, USA, 2–6 February 2007. [Google Scholar]
- Wosicka, H.; Cal, K. Targeting to the hair follicles: Current status and potential. J. Dermatol. Sci. 2010, 57, 83–89. [Google Scholar] [CrossRef]
- Balañá, M.E.; Charreau, H.E.; Leirós, G.J. Epidermal stem cells and skin tissue engineering in hair follicle regeneration. World J. Stem Cells 2015, 7, 711. [Google Scholar] [CrossRef]
- Liakou, A.I.; Theodorakis, M.J.; Melnik, B.C.; Pappas, A.; Zouboulis, C.C. Nutritional clinical studies in dermatology. J. Drugs Dermatol. 2013, 12, 1104–1109. [Google Scholar] [PubMed]
- Kwon, H.-K.; Song, M.-J.; Lee, H.-J.; Park, T.-S.; Kim, M.; Park, H.-J. Pediococcus pentosaceus-Fermented Cordyceps militaris Inhibits Inflammatory Reactions and Alleviates Contact Dermatitis. Int. J. Mol. Sci. 2018, 19, 3504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.-N.; Park, D.K.; Park, H.-J. Hair growth-promoting activity of hot water extract of Thuja orientalis. BMC Complement. Altern. Med. 2013, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Soma, T.; Fujiwara, S.; Shirakata, Y.; Hashimoto, K.; Kishimoto, J. Hair-inducing ability of human dermal papilla cells cultured under Wnt/beta-catenin signalling activation. Exp. Dermatol. 2012, 21, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Jahoda, C.A.B.; Reynolds, A.J. Dermal-epidermal interactions-Adult follicle-derived cell populations and hair growth. Dermatol. Clin. 1996, 14, 573–583. [Google Scholar] [CrossRef]
- Paus, R.; Christoph, T.; Muller-Rover, S. Immunology of the hair follicle: A short journey into terra incognita. J. Investig. Dermatol. Symp. Proc. 1999, 4, 226–234. [Google Scholar] [CrossRef]
- Paus, R.; Foitzik, K. In search of the “hair cycle clock”: A guided tour. Differentiation 2004, 72, 489–511. [Google Scholar] [CrossRef]
- Richardson, G.D.; Arnott, E.C.; Whitehouse, C.J.; Lawrence, C.M.; Reynolds, A.J.; Hole, N.; Jahoda, C.A. Plasticity of rodent and human hair follicle dermal cells: Implications for cell therapy and tissue engineering. J. Investig. Dermatol. Symp. Proc. 2005, 10, 180–183. [Google Scholar]
- Sari, A.R.P.; Rufaut, N.W.; Jones, L.N.; Sinclair, R.D. Characterization of ovine dermal papilla cell aggregation. Int. J. Trichol. 2016, 8, 121. [Google Scholar] [CrossRef]
- MuÈller-RoÈver, S.; Foitzik, K.; Paus, R.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; McKay, I.A.; Stenn, K.S. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef]
- Tiede, S.; Kloepper, J.; Whiting, D.; Paus, R. The ‘follicular trochanter’: An epithelial compartment of the human hair follicle bulge region in need of further characterization. Br. J. Dermatol. 2007, 157, 1013–1016. [Google Scholar] [CrossRef]
- Johnstone, M.A.; Albert, D.M. Prostaglandin-induced hair growth. Surv. Ophthalmol. 2002, 47, S185–S202. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Wortsman, J.; Plonka, P.M.; Schallreuter, K.U.; Paus, R.; Tobin, D.J. Hair follicle pigmentation. J. Investig. Dermatol. 2005, 124, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Hattori, M. Regulation mechanisms of hair growth. In Normal and Abnormal Epidermal Differentiation; Karger Publishers: Basel, Switzerland, 1983; Volume 11, pp. 159–170. [Google Scholar]
- Slominski, A.; Paus, R. Melanogenesis is coupled to murine anagen: Toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J. Investig. Dermatol. 1993, 101, 90–97. [Google Scholar] [CrossRef]
- Aillon, K.L.; Xie, Y.; El-Gendy, N.; Berkland, C.J.; Forrest, M.L. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv. Drug Deliv. Rev. 2009, 61, 457–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705. [Google Scholar] [CrossRef]
- Cruz, C.F.; Martins, M.; Egipto, J.; Osório, H.; Ribeiro, A.; Cavaco-Paulo, A. Changing the shape of hair with keratin peptides. Rsc Adv. 2017, 7, 51581–51592. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Gordon, S.W.; Nixon, A.J.; Bawden, C.S.; Rogers, M.A.; Wildermoth, J.E.; Maqbool, N.J.; Pearson, A.J. Expression patterns of keratin intermediate filament and keratin associated protein genes in wool follicles. Differentiation 2009, 77, 307–316. [Google Scholar] [CrossRef]
- Rogers, G.E. Hair follicle differentiation and regulation. Int. J. Dev. Biol. 2003, 48, 163–170. [Google Scholar] [CrossRef]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell 2010, 18, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, S.; Collin, C.; Langbein, L.; Schweizer, J.; Gautier, B.; Bernard, B. Hair keratin pattern in human hair follicles grown in vitro. Exp. Dermatol. 2003, 12, 160–164. [Google Scholar] [CrossRef]
- Roh, C.; Tao, Q.; Lyle, S. Dermal papilla-induced hair differentiation of adult epithelial stem cells from human skin. Physiol. Genom. 2004, 19, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keough, R.A. An Investigation of Hair Follicle Cell Immortalisation and Hair Keratin Gene Regulation/Rebbeca Anne Keough. Ph.D. Thesis, Department of Biochemistry, University of Adelaide, Adelaide, Australia, 1995. [Google Scholar]
- Schweizer, J.; Langbein, L.; Rogers, M.A.; Winter, H. Hair follicle-specific keratins and their diseases. Exp. Cell Res. 2007, 313, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Langbein, L.; Yoshida, H.; Praetzel-Wunder, S.; Parry, D.A.; Schweizer, J. The keratins of the human beard hair medulla: The riddle in the middle. J. Investig. Dermatol. 2010, 130, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Zlotogorski, A. Keratins: The hair shaft’s backbone revealed. Exp. Dermatol. 2015, 24, 416–417. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Siddiqui, I.A.; Syed, D.N.; Adhami, V.M.; Liovic, M.; Mukhtar, H. Keratin gene mutations in disorders of human skin and its appendages. Arch. Biochem. Biophys. 2011, 508, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Fukumoto, S.; Hsieh, C.-M.; Maemura, K.; Layne, M.D.; Yet, S.-F.; Lee, K.-H.; Matsui, T.; Rosenzweig, A.; Taylor, W.G.; Rubin, J.S. Akt participation in the Wnt signaling pathway through Dishevelled. J. Biol. Chem. 2001, 276, 17479–17483. [Google Scholar] [CrossRef]
- Kastan, M.B.; Bartek, J. Cell-cycle checkpoints and cancer. Nature 2004, 432, 316. [Google Scholar] [CrossRef]
- Sherr, C.J. Cancer cell cycles. Science 1996, 274, 1672–1677. [Google Scholar] [CrossRef]
- Prall, O.W.; Sarcevic, B.; Musgrove, E.A.; Watts, C.K.; Sutherland, R.L. Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibitor association with cyclin E-Cdk2. J. Biol. Chem. 1997, 272, 10882–10894. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Walker, C. Cyclins and cell cycle checkpoints. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef]
- Williams, D.; Profeta, K.; Stenn, K. Isolation and culture of follicular papillae from murine vibrissae: An introductory approach. Br. J. Dermatol. 1994, 130, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Liu, R.Q.; Lu, Y.G.; Zhu, T.Y.; Cheng, B.; Men, X. Enzyme digestion to isolate and culture human scalp dermal papilla cells: A more efficient method. Arch. Dermatol. Res. 2005, 297, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Oakley, B.R.; Kirsch, D.R.; Morris, N.R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal. Biochem. 1980, 105, 361–363. [Google Scholar] [CrossRef]
- Schweizer, J.; Bowden, P.E.; Coulombe, P.A.; Langbein, L.; Lane, E.B.; Magin, T.M.; Maltais, L.; Omary, M.B.; Parry, D.A.; Rogers, M.A. New consensus nomenclature for mammalian keratins. J. Cell Biol. 2006, 174, 169–174. [Google Scholar] [CrossRef] [Green Version]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Kwon, H.-K.; Kim, H.S.; Kim, M.I.; Park, H.-J. Hair Growth Promoting Effect of 4HGF Encapsulated with PGA Nanoparticles (PGA-4HGF) by β-Catenin Activation and Its Related Cell Cycle Molecules. Int. J. Mol. Sci. 2019, 20, 3447. https://doi.org/10.3390/ijms20143447
Lee H-J, Kwon H-K, Kim HS, Kim MI, Park H-J. Hair Growth Promoting Effect of 4HGF Encapsulated with PGA Nanoparticles (PGA-4HGF) by β-Catenin Activation and Its Related Cell Cycle Molecules. International Journal of Molecular Sciences. 2019; 20(14):3447. https://doi.org/10.3390/ijms20143447
Chicago/Turabian StyleLee, Hye-Ji, Ha-Kyoung Kwon, Hye Su Kim, Moon Il Kim, and Hye-Jin Park. 2019. "Hair Growth Promoting Effect of 4HGF Encapsulated with PGA Nanoparticles (PGA-4HGF) by β-Catenin Activation and Its Related Cell Cycle Molecules" International Journal of Molecular Sciences 20, no. 14: 3447. https://doi.org/10.3390/ijms20143447