Research Progress and Perspective on Drought Stress in Legumes: A Review
Abstract
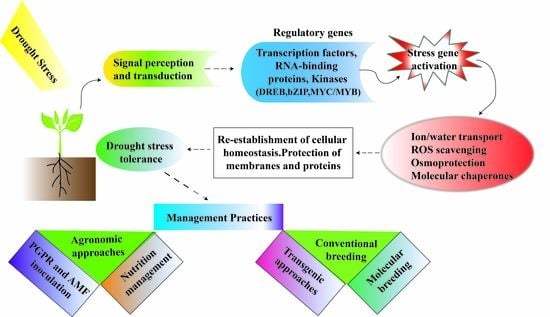
:1. Introduction
2. Effect of Drought Stress (DS) on Legumes
3. Tolerance Mechanisms
3.1. Drought Escape and Avoidance
3.2. Solute Accumulation
3.3. Antioxidant Defense
3.4. Hormone Regulation
3.5. Potential Traits for Screening Legumes for Drought Resistance
4. Management Strategies
4.1. Agronomic Strategies
4.1.1. Planting Time and Plant Geometry
4.1.2. Nutrient Management
4.2. Plant Growth-Promoting Rhizobacteria and Arbuscular Mycorrhizal Fungal Inoculation
5. Development of DS-Tolerant Legumes Using Molecular and Biotechnological Approaches
5.1. Breeding Approach
5.2. Quantitative Trait Loci (QTL) for Drought Tolerance
5.3. Biotechnology and Functional Genomics
5.4. OMICS-Based Approaches
5.5. CRISPR/Cas9: Powerful Tool for Genome Editing (GE)
6. Conclusions and Future Research Perspectives
Funding
Conflicts of Interest
Abbreviations
| GA | Gibberellin |
| ABA | Abscisic acid |
| PPDK | Pyruvate, phosphate dikinase |
| JA | Jasmonic acid |
| GB | Glycine betaine |
| ROS | Reactive oxygen species |
| GR | Glutathione reductase |
| GPX | Glutathione peroxidases |
| APX | Ascorbate peroxidase |
| SOD | Superoxide dismutase |
| GST | Glutathione S-transferases |
| MDHAR | Monodehydroascorbate reductase |
| CAT | Catalase |
| sRWC | Relative water content |
| WUE | Water use efficiency |
| PGPR | Plant growth promoting rhizobacteria |
| AMF | Arbuscular mycorrhizal fungi |
| WGS | Whole genome sequencing |
| SNP | Single-nucleotide polymorphism |
| SSRs | Simple sequence repeats |
| NGS | Next-generation sequencing |
| MDA | Malondialdehyde |
| GE | Genome editing |
References
- Xu, J.; Yuan, Y.; Xu, Y.; Zhang, G.; Guo, X.; Wu, F.; Wang, Q.; Rong, T.; Pan, G.; Cao, M.; et al. Identification of candidate genes for drought tolerance by whole-genome resequencing in maize. BMC Plant Biol. 2014, 14, 83. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Wang, M.; Shah, L.; Lu, S.; Wang, X.; Ma, C. Unraveling field crops sensitivity to heat stress: Mechanisms, approaches, and future prospects. Agronomy 2018, 8, 128. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Wang, M.; Ali, A.; Cheng, A.; Wang, X.; Ma, C. Grain legumes and fear of salt stress: Focus on mechanisms and management strategies. Int. J. Mol. Sci. 2019, 20, 799. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.A.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Growth and developmental responses of crop plants under drought stress: A review. Zemdirbyste-Agriculture 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-allah, S.; Tanveer, M. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. J. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Chowdhury, J.A.; Karim, M.A.; Khaliq, Q.A.; Ahmed, A.U.; Khan, M.S.A. Effect of drought stress on gas exchange characteristics of four soybean genotypes. Bangladesh J. Agr. Res. 2016, 41, 195–205. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Todaka, D.; Zhao, Y.; Yoshida, T.; Kudo, M.; Kidokoro, S.; Mizoi, J.; Kodaira, K. Temporal and spatial changes in gene expression, metabolite accumulation and phytohormone content in rice seedlings grown under drought stress conditions. Plant J. 2017, 90, 61–78. [Google Scholar] [CrossRef]
- Siddique, K.H.M.; Loss, S.P.; Regan, K.L.; Jettner, R.L. Adaptation and seed yield of cool season grain legumes in Mediterranean environments of South-Western Australia. Aust. J. Agric. Res. 1999, 50, 375–388. [Google Scholar] [CrossRef]
- Rubiales, D.; Mikic, A. Introduction: Legumes in sustainable agriculture. CRC Crit. Rev. Plant Sci. 2015, 34, 2–3. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Update on legume utilization legumes: Importance and constraints to greater use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef]
- Micheletto, S.; Rodriguez-uribe, L.; Hernandez, R.; Richins, R.D.; Curry, J.; Connell, M.A.O. Comparative transcript profiling in roots of Phaseolus acutifolius and P. vulgaris under water deficit stress. Plant Sci. 2007, 173, 510–520. [Google Scholar] [CrossRef]
- Fang, X.; Turner, N.C.; Yan, G.; Li, F.; Siddique, K.H.M. Flower numbers, pod production, pollen viability, and pistil function are reduced and flower and pod abortion increased in chickpea (Cicer arietinum L.) under terminal drought. J. Exp. Bot. 2010, 61, 335–345. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S. Drought stress in grain legumes during reproduction and grain filling. J. Agron. Crop Sci. 2016, 203, 81–102. [Google Scholar] [CrossRef]
- Mittal, N.; Mishra, A.; Singh, R.; Kumar, P. Assessing future changes in seasonal climatic extremes in the Ganges river basin using an ensemble of regional climate models. Clim. Change 2014, 123, 273–286. [Google Scholar] [CrossRef]
- Delmer, D.P. Agriculture in the developing world: Connecting innovations in plant research to downstream applications. Proc. Natl. Acad. Sci. USA 2005, 102, 15739–15746. [Google Scholar] [CrossRef] [Green Version]
- Pushpavalli, R.; Zaman-allah, M.; Turner, N.C.; Baddam, R.; Rao, M.V.; Vadez, V. Higher flower and seed number leads to higher yield under water stress conditions imposed during reproduction in chickpea. Funct. Plant Biol. 2015, 42, 162–174. [Google Scholar] [CrossRef]
- Siddique, K.H.M.; Regan, K.L.; Tennant, D.; Thomson, B.D. Water use and water use efficiency of cool season grain legumes in low rainfall Mediterranean-type environments. Eur. J. Agron. 2001, 15, 267–280. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Mardfar, R.A. Effects of limited irrigation on growth and grain yield of common bean. J. Plant Sci. 2008, 3, 230–235. [Google Scholar]
- Demirta, C.; Yazgan, S.; Candogan, B.N.; Sincik, M. Quality and yield response of soybean (Glycine max L. Merrill) to drought stress in sub–humid environment. Afr. J. Biotechnol. 2010, 9, 6873–6881. [Google Scholar]
- Baroowa, B.; Gogoi, N. Biochemical changes in two Vigna spp. during drought and subsequent recovery. Indian J. Plant Physiol. 2013, 18, 319–325. [Google Scholar] [CrossRef]
- Ghassemi-golezani, K.; Ghassemi, S.; Bandehhagh, A. Effects of water supply on field performance of chickpea (Cicer arietinum L.) cultivars. Int. J. Agron. Plant Prod. 2013, 4, 94–97. [Google Scholar]
- Ulemale, C.S.; Mate, S.N.; Deshmukh, D.V. Physiological indices for drought tolerance in chickpea (Cicer arietinum L.). World J. Agric. Sci. 2013, 9, 123–131. [Google Scholar] [CrossRef]
- Miyauchi, Y.; Isoda, A.; Li, Z.; Wang, P. Soybean cultivation on desert sand using drip irrigation with mulch. Plant Prod. Sci. 2012, 15, 310–316. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; De Groot, S.; Soole, K.; Langridge, P. Early flowering as a drought escape mechanism in plants: How can it aid wheat production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef]
- Kamanga, R.M.; Mbega, E.; Ndakidemi, P. Drought tolerance mechanisms in plants: physiological responses associated with water deficit stress in Solanum lycopersicum. Adv. Crop Sci. Technol. 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Bechtold, U. Plant life in extreme environments: How do you improve drought tolerance? Front. Plant Sci. 2018, 9, 543. [Google Scholar] [CrossRef]
- Mondal, M.M.A.; Fakir, S.A.; Juraimi, A.S.; Hakim, M.A.; Islam, M.M.; Shamsuddoha, A.T.M. Effects of flowering behavior and pod maturity synchrony on yield of mungbean [Vigna radiata (L.) Wilczek]. Aust. J. Crop Sci. 2011, 5, 945–953. [Google Scholar]
- Zlatev, Z.; Lidon, F.C. An overview on drought induced changes in plant growth, water relations and photosynthesis. Emirates J. Food Agric. 2012, 24, 57–72. [Google Scholar]
- Samarah, N.H.; Haddad, N.; Alqudah, A.M. Yield potential evaluation in chickpea genotypes under late terminal drought in relation to the length of reproductive stage. Ital. J. Agron. 2009, 3, 111–117. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Oliver, M.J. Drought or/and heat-stress effects on seed filling in food crops: Impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef]
- Croser, J.; Ahmad, F.; Siddique, K.H.M. Utilisation of wild Cicer in chickpea improvement-progress, constraints, and prospects. Aust. J. Agric. Res. 2003, 54, 429–444. [Google Scholar] [CrossRef]
- Liu, F.; Jensen, C.R.; Andersen, M.N. Pod Set Related to photosynthetic rate and endogenous ABA in soybeans subjected to different water regimes and exogenous ABA and BA at early reproductive stages. Ann. Bot. 2004, 94, 405–411. [Google Scholar] [CrossRef]
- Vadez, V.; Berger, J.D.; Warkentin, T.; Asseng, S.; Ratnakumar, P.; Rao, K.P.C.; Gaur, P.M. Adaptation of grain legumes to climate change: A review. Agron. Sustain. Dev. 2012, 32, 31–44. [Google Scholar] [CrossRef]
- Andersen, M.N.; Asch, F.; Wu, Y.; Jensen, C.R.; Næsted, H.; Mogensen, V.O.; Koch, K.E. Soluble invertase expression is an early target of drought stress during the critical, abortion-sensitive phase of young ovary development in maize. Plant Physiol. 2015, 130, 591–604. [Google Scholar] [CrossRef]
- Heatherly, L.G. Drought stress and irrigation effects on germination of harvested soybean seed. Crop Sci. 1993, 33, 777–781. [Google Scholar] [CrossRef]
- Awari, V.R.; Mate, S.N. Effect of drought stress on early seedling growth of chickpea (Cicer arietinum L.) genotypes. Life Sci. Int. Res. J. 2015, 2, 356–361. [Google Scholar]
- Li, P.; Zhang, Y.; Wu, X.; Liu, Y. Drought stress impact on leaf proteome variations of faba bean (Vicia faba L.) in the Qinghai–Tibet Plateau of China. 3 Biotech 2018, 8, 110. [Google Scholar] [CrossRef]
- Andrianasolo, F.N.; Debaeke, P.; Champolivier, L.; Maury, P. Analysis and modelling of the factors controlling seed oil concentration in sunflower: A review. OCL 2016, 23, D206. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Fujita, K. Effects of drought stress on photosynthetic gas exchange, chlorophyll fluorescence and stem diameter of soybean plants. Biol. Plant. 2006, 50, 138–141. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Pan, D.; Zhang, Y.; Luo, B.; Ji, J. Effects of drought stress on photosynthesis and chlorophyll fluorescence images of soybean (Glycine max L.) seedlings. Int. J. Agric. Biol. Eng. 2018, 11, 196–201. [Google Scholar] [CrossRef]
- Hao, X.; Li, P.; Feng, Y.; Han, X.; Gao, J.; Lin, E.; Han, Y. Effects of fully open-air [CO2] elevation on leaf photosynthesis and ultrastructure of Isatis indigotica Fort. PLoS ONE 2013, 8, e74600. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-khaishany, M.Y.; Al-qutami, M.A. Response of different genotypes of faba bean plant to drought stress. Int. J. Mol. Sci. 2015, 16, 10214–10227. [Google Scholar] [CrossRef]
- Abid, G.; Mahmoud, M.; Mingeot, D.; Aouida, M. Effect of drought stress on chlorophyll fluorescence, antioxidant enzyme activities and gene expression patterns in faba bean (Vicia faba L.). Arch. Agron. Soil Sci. 2016, 63, 536–552. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Makbul, S.; Güler, N.S.; Durmuş, N.; Güven, S. Changes in anatomical and physiological parameters of soybean under drought stress. Turk. J. Botany 2011, 35, 369–377. [Google Scholar] [CrossRef]
- Mak, M.; Babla, M.; Xu, S.; Carrigan, A.O.; Liu, X.; Gong, Y.; Holford, P.; Chen, Z. Leaf mesophyll K+, H+ and Ca2+ fluxes are involved in drought-induced decrease in photosynthesis and stomatal closure in soybean. Environ. Exp. Bot. 2014, 98, 1–12. [Google Scholar] [CrossRef]
- Mutava, R.N.; Jebakumar, S.; Prince, K.; Hasan, N.; Song, L.; Valliyodan, B.; Chen, W.; Nguyen, H.T. Understanding abiotic stress tolerance mechanisms in soybean: A comparative evaluation of soybean response to drought and flooding stress. Plant Physiol. Biochem. 2014, 109–120. [Google Scholar] [CrossRef]
- Abdel, C.G.; Stutzel, H. Effects of water stress on Epidermis and stomata population of sixteen water stressed and irrigated barley (Hordeum vulgare L.) genotypes. Euphrates J. Agric. Sci. 2016, 8, 48–62. [Google Scholar]
- Atti, S.; Bonnell, R.; Smith, D.; Prasher, S. Response of an indeterminate soybean {Glycine Max (L.) Merr} to chronic water deficit during reproductive development under greenhouse conditions. Can. Water Resour. J. 2004, 29, 209–222. [Google Scholar] [CrossRef]
- De Souza, P.I.; Egli, D.B.; Bruening, W.P. Water stress during seed filling and leaf senescence in soybean. Agron. J. 1997, 89, 807–812. [Google Scholar] [CrossRef]
- Liu, F.; Andersen, M.N.; Jacobsen, S.; Jensen, C.R. Stomatal control and water use efficiency of soybean (Glycine max L. Merr.) during progressive soil drying. Environ. Exp. Bot. 2005, 54, 33–40. [Google Scholar] [CrossRef]
- Farooq, M.; Farooq, M.; Hussain, M.; Siddique, K.H.M. drought stress in wheat during flowering and grain- filling periods. Crit. Rev. Plant Sci. 2014, 33, 331–349. [Google Scholar] [CrossRef]
- Phillips, B.B.; Shaw, R.F.; Holland, M.J.; Fry, E.L.; Bardgett, R.D.; Bullock, J.M.; Osborne, J.L. Drought reduces floral resources for pollinators. Glob. Chang. Biol. 2018, 24, 3226–3235. [Google Scholar] [CrossRef] [PubMed]
- Gusmao, M.; Siddique, K.H.M.; Flower, K.; Nesbitt, H.; Veneklaas, E.J. Water deficit during the reproductive period of grass Pea (Lathyrus sativus L.) reduced grain yield but maintained seed size. J. Agron. Crop Sci. 2012, 198, 430–441. [Google Scholar] [CrossRef]
- Desclaux, D.; Roumet, P. Identification of soybean plant characteristics that indicate the timing of drought stress. Crop Sci. 2000, 40, 716–722. [Google Scholar] [CrossRef]
- Li, D.; Liu, H.; Qiao, Y.; Wang, Y.; Cai, Z.; Dong, B.; Shi, C.; Liu, Y.; Li, X.; Liu, M. Effects of elevated CO2 on the growth, seed yield, and water use efficiency of soybean (Glycine max. L.) under drought stress. Agric. Water Manag. 2013, 129, 105–112. [Google Scholar] [CrossRef]
- Kokubun, M.; Shimada, S.; Takahashi, M. Flower abortion caused by preanthesis water deficit is not attributed to impairment of pollen in soybean. Crop Sci. 2001, 41, 1517–1521. [Google Scholar] [CrossRef]
- He, J.; Du, Y.; Wang, T.; Turner, N.C.; Yang, R.; Jin, Y.; Xi, Y.; Zhang, C.; Cui, T.; Fang, X.; et al. Conserved water use improves the yield performance of soybean. Agric. Water Manag. 2016. [Google Scholar] [CrossRef]
- Maleki, A.; Naderi, A.; Naseri, R.; Fathi, A.; Bahamin, S.; Maleki, R. Physiological performance of soybean cultivars under drought stress. Bull. Environ. Pharmacol. Life Sci. 2013, 2, 38–44. [Google Scholar]
- José, A.; Rodrigues, J.; Filho, M.; Rodrigues, C.; Sales, G.; Célia, R.; Pires, D.M.; Machado, E.C. Source-sink relationships in two soybean cultivars with indeterminate growth under water deficit. Bragantia 2018, 77, 23–35. [Google Scholar] [Green Version]
- Meckel, L.; Egli, D.B.; Phillips, R.E.; Radcliffe, D.; Leggett, J.E. Effect of moisture stress on seed growth in soybeans. Agron. J. 1984, 76, 647–650. [Google Scholar] [CrossRef]
- Rotundo, L.; Westgate, M.E. Meta-analysis of environmental effects on soybean seed composition. F. Crop. Res. 2009, 110, 147–156. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, J.; Jiang, S.; Ning, S.; Liu, L. Quantitative response of soybean development and yield to drought stress during different growth stages in the Huaibei Plain, China. Agronomy 2018, 8, 97. [Google Scholar] [CrossRef]
- Baroowa, B.; Gogoi, N. Biochemical changes in black gram and green gram genotypes after imposition of drought stress. J. Food Legum. 2014, 27, 350–353. [Google Scholar]
- Kobraee, S.; Shamsi, K.; Rasekhi, B. Soybean production under water deficit conditions. Sch. Res. Libr. 2011, 2, 423–434. [Google Scholar]
- Samarah, N.H.; Mullen, R.E.; Cianzio, S.R.; Scott, P. Dehydrin-like proteins in soybean seeds in response to drought stress during seed filling. Crop Sci. 2006, 46, 2141–2150. [Google Scholar] [CrossRef]
- Nayyar, H.; Kaur, S.; Singh, S.; Upadhyaya, H.D. Differential sensitivity of Desi (small-seeded) and Kabuli (large-seeded) chickpea genotypes to water stress during seed filling: effects on accumulation of seed reserves and yield. J. Sci. Food Agric. 2006, 2082, 2076–2082. [Google Scholar] [CrossRef]
- Varshney, R.K.; Thudi, M.; Nayak, S.N.; Gaur, P.M.; Kashiwagi, J.; Krishnamurthy, L.; Jaganathan, D.; Koppolu, J.; Bohra, A.; Tripathi, S.; et al. Genetic dissection of drought tolerance in chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2014, 127, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Ogbonnaya, C.I.; Sarr, B.; Brou, C.; Diouf, O.; Diop, N.N.; Roy-Macauley, H. Selection of cowpea genotypes in hydroponics, pots, and field for drought tolerance. Crop Sci. 2003, 43, 1114–1120. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Suliman, A.S.H. Effect of water stress applied at different stages of growth on seed yield and water-use efficiency of Cowpea. Agric. Biol. J. North Am. 2010, 1, 534–540. [Google Scholar]
- Kyei-boahen, S.; Savala, C.E.N.; Chikoye, D.; Abaidoo, R.; Kyei-boahen, S. Growth and yield responses of cowpea to inoculation and phosphorus fertilization in different environments. Front. Plant Sci. 2017, 8, 646. [Google Scholar] [CrossRef]
- Martinez, J.P.; Silva, H.; Ledent, J.F.; Pinto, M. Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). Eur. J. Agron. 2007, 26, 30–38. [Google Scholar] [CrossRef]
- Ghanbari, A.A.; Mousavi, S.H.; Mousapour, A.; Rao, I. Effects of water stress on leaves and seeds of bean (Phaseolus vulgaris L.). Turkish J. F. Crop. 2013, 18, 73–77. [Google Scholar]
- Rosales-Serna, R.; Kohashi-Shibata, J.; Acosta-Gallegos, J.A.; Trejo-López, C.; Ortiz-Cereceres, J.; Kelly, J.D. Biomass distribution, maturity acceleration and yield in drought-stressed common bean cultivars. F. Crop. Res. 2004, 85, 203–211. [Google Scholar] [CrossRef]
- Nam, N.H.; Chauhan, Y.S.; Johansen, C. Effect of timing of drought stress on growth and grain yield of extra-short-duration pigeonpea lines. J. Agric. Sci. 2001, 136, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Al, R.; Dahanayaka, N.; Ugs, A.; Wdrj, R.; Utd, R. Effect of water stress on growth and yield of mung bean (Vigna radiata L.). Trop. Agric. Res. Ext. 2012, 14, 2–6. [Google Scholar] [CrossRef]
- Ahmad, A.; Selim, M.M.; Alderfasi, A.A.; Afzal, M. Effect of drought stress on mungbean (Vigna radiata L.) under arid climatic conditions of Saudi Arabia. Ecosyst. Sustain. Dev. 2015, 192, 185–193. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Hosseinzadeh-Mahootchy, A. Changes in seed vigour of faba bean (Vicia faba L.) cultivars during development and maturity. Seed Sci. Technol. 2009, 37, 713–720. [Google Scholar] [CrossRef]
- Shrestha, R.A.; Turner, N.C.A.; Siddique, K.H.M.A.; Turner, D.W.B.; Speijers, J.C. A water deficit during pod development in lentils reduces flower and pod numbers but not seed size. Aust. J. Agr. Res. 2006, 57, 427–438. [Google Scholar] [CrossRef]
- Allahmoradi, P.; Mansourifar, C.; Saiedi, M.; Jalali Honarmand, S. Effect of different water deficiency levels on some antioxidants at different growth stages of lentil (Lens culinaris L.). Adv. Environ. Biol. 2013, 7, 535–543. [Google Scholar]
- Siddique, K.H.M.; Walton, G.H.; Seymour, M. A comparison of seed yields of winter grain legumes in Western Australia. Aust. J. Exp. Agric. 1993, 33, 15–22. [Google Scholar] [CrossRef]
- Hall, A.E. Phenotyping cowpeas for adaptation to drought. Front. Physiol. 2012, 3, 155. [Google Scholar] [CrossRef] [PubMed]
- Thomson, B.D.; Siddique, K.H.M.; Barr, M.D.; Wilson, J.M. Grain legume species in low rainfall Mediterranean-type environments I. Phenology and seed yield. F. Crop. Res. 1997, 54, 173–187. [Google Scholar] [CrossRef]
- Calvache, M.; Reichardt, K.; Bacchp, O.O.S. Deficit irrigation at different growth stages of the common bean. Sci. Agric. 1997, 54, 1–16. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Ashraf, M.Y. Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Crop Sci. 2011, 5, 764–777. [Google Scholar]
- Majumdar, R.; Barchi, B.; Turlapati, S.A.; Gagne, M. Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: The pathway is regulated at the post-transcriptional level. Front. Plant Sci. 2016, 7, 78. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solanki, J.K.; Sarangi, S.K. Effect of drought stress on proline accumulation in peanut genotypes. Int. J. Adv. Res. 2015, 2, 301–309. [Google Scholar]
- Shinde, S.; Villamor, J.G.; Lin, W.; Sharma, S.; Verslues, P.E. Proline co-ordination with fatty acid synthesis and redox metabolism of chloroplast and mitochondria. Plant Physiol. 2016, 172, 1074–1088. [Google Scholar] [CrossRef] [PubMed]
- Ramanjulu, S.; Bartels, D. Drought and desiccation induced modulation of gene. Plant Cell Environ. 2002, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Jensen, R.C.; Bohnert, H. Mannitol protects against oxidation by hydroxyl radicals. Plant Physiol. 1997, 115, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Bhauso, T.D.; Radhakrishnan, T.; Kumar, A.; Mishra, G.P.; Dobaria, J.R. Overexpression of bacterial mtlD gene in peanut improves drought tolerance through accumulation of mannitol. Sci. World J. 2014, 10. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Abdellatif, Y.M.R. Effect of maltose and trehalose on growth, yield and some biochemical components of wheat plant under water stress. Ann. Agric. Sci. 2016, 61, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Khater, M.A.; Dawood, M.G.; Sadak, M.S.; Shalaby, M.A.F.; And, M.E.E.-A.; El-Din, K.G. Enhancement the performance of cowpea plants grown under drought conditions via trehalose application. Middle East J. Agric. Res. 2018, 7, 782–800. [Google Scholar]
- Amede, T.; Schubert, S. Mechanisms of drought resistance in grain legumes i: osmotic adjustment. Ethiop. J. Sci. 2003, 26, 37–46. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Smirnoff, N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Farnese, F.S.; Menezes-silva, P.E.; Gusman, G.S.; Oliveira, J.A. When bad guys become good ones: The key role of reactive oxygen species and nitric oxide in the plant responses to abiotic stress. Front. Plant Sci. 2016, 7, 471. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 1–22. [Google Scholar] [CrossRef]
- Al Hassan, M.; Chaura, J.; Donat-torres, P.; Boscaiu, M. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants 2017, 9, plx009. [Google Scholar] [CrossRef]
- Sahitya, U.L.; Krishna, M.S.R.; Prasad, G.S.; Kasim, D.P.; Deepthi, R.S. Seed antioxidants interplay with drought stress tolerance indices in chilli (Capsicum annuum L.) Seedlings. Biomed Res. Int. 2018, 2018, 14. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Aditya, M.; Dey, N.; Banik, N. Antioxidant signaling and redox regulation in drought- and salinity-stressed plants. In Drought Stress Tolerance in Plants; Springer: Berlin, Germany, 2016; Volume 1, ISBN 9783319288994. [Google Scholar]
- Noctor, G.; Veljovic-jovanovic, S.; Foyer, C.H. Peroxide processing in photosynthesis: antioxidant coupling and redox signalling. R. Soc. 2000, 355, 1465–1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoz, T.; Castagnara, D. Peroxidase activity as an indicator of water deficit tolerance in soybean cultivars. Biosci. J. 2013, 29, 1664–1671. [Google Scholar]
- Yasar, F.; Uzal, O.; Yasar, T.O. Investigation of the relationship between the tolerance to drought stress levels and antioxidant enzyme activities in green bean (Phaseolus Vulgaris L.) genotypes. Afr. J. Agric. Res. 2013, 8, 5759–5763. [Google Scholar] [CrossRef]
- Mittler, R.; Zilinskas, B.A. drought stress and following recovery from drought. Plant J. 1994, 5, 397–405. [Google Scholar] [CrossRef]
- Osman, H.S. Enhancing antioxidant yield relationship of pea plant under drought at different growth stages by exogenously applied glycine betaine and proline. Ann. Agric. Sci. 2015, 60, 389–402. [Google Scholar] [CrossRef]
- Guler, N.S.; Pehlivan, N. Exogenous low-dose hydrogen peroxide enhances drought tolerance of soybean (Glycine max L.) through inducing antioxidant system. Acta Biol. Hung. 2016, 67, 169–183. [Google Scholar] [CrossRef]
- Patel, P.K.; Hemantaranjan, A.; Sarma, B.K.; Singh, R. Growth and antioxidant system under drought stress in chickpea (Cicer arietinum L.) as sustained by salicylic acid. J. Stress Physiol. Biochem. 2011, 7, 130–144. [Google Scholar]
- Saglam, A.; Saruhan, N.; Terzi, R.; Kadioglu, A. The relations between antioxidant enzymes and chlorophyll fluorescence parameters in common bean cultivars differing in sensitivity to drought stress. Russ. J. Plant Physiol. 2011, 58, 60–68. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Yadav, S.K. Comparative study on biochemical parameters and antioxidant enzymes in a drought tolerant and a sensitive variety of horsegram under drought stress. Am. J. Plant Physilol. 2012, 7, 17–29. [Google Scholar]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef] [PubMed]
- Bielach, A.; Hrtyan, M.; Tognetti, V.B. Plants under stress: Involvement of Auxin and Cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar] [CrossRef] [PubMed]
- Weyers, J.D.B.; Paterson, N.W. Plant hormones and the control of physiological processes. New Phytol. 2001, 152, 375–407. [Google Scholar] [CrossRef] [Green Version]
- Hartung, W.; Sauter, A.; Hose, E. Abscisic acid in the xylem: Where does it come from, where does it go to? J. Exp. Bot. 2002, 53, 27–32. [Google Scholar] [CrossRef]
- Miyashita, K.; Tanakamaru, S.; Maitani, T.; Kimura, K. Recovery responses of photosynthesis, transpiration, and stomatal conductance in kidney bean following drought stress. Environ. Exp. Bot. 2005, 53, 205–214. [Google Scholar] [CrossRef]
- Merilo, E.; Pirko, J.; Kristiina, L.; Omid, M.; Hanna, H.; Hannes, K.; Mikael, B. Abscisic acid transport and homeostasis in the context of stomatal regulation. Mol. Plant 2015, 8, 1321–1333. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Martinoia, E.; Geisler, M. Plant hormone transporters: What we know and what we would like to know. BMC Biol. 2017, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.H.; Hanan, H.L. Improvement of drought tolerance of soybean plants by using methyl jasmonate. Physiol. Mol. Biol. Plants 2017, 23, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Xia, H.; Lu, B.-R. Editorial: Crop breeding for drought resistance. Front. Plant Sci. 2019, 10, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Vadez, V.; Rao, S.; Kholova, J.; Krishnamurthy, L.; Kashiwagi, J.; Ratnakumar, P. Root research for drought tolerance in legumes: Quo vadis? J. Food Legum. 2008, 21, 77–85. [Google Scholar]
- Sofi, P.A.; Djanaguiraman, M.; Siddique, K.H.M.; Prasad, P.V.V. Reproductive fitness in common bean (Phaseolus vulgaris L.) under drought stress is associated with root length and volume. Indian J. Plant Physiol. 2018, 23, 796–809. [Google Scholar] [CrossRef]
- Kunert, K.J.; Vorster, B.J.; Fenta, B.A.; Kibido, T.; Dionisio, G.; Foyer, C.H. Drought stress responses in soybean roots and nodules. Front. Plant Sci. 2016, 7, 1015. [Google Scholar] [CrossRef]
- Ramamoorthy, P.; Lakshmanan, K.; Upadhyaya, H.D.; Vadez, V.; Varshney, R.K. Root traits confer grain yield advantages under terminal drought in chickpea (Cicer arietinum L.). F. Crop. Res. 2017, 201, 146–161. [Google Scholar] [CrossRef]
- Bangar, P.; Chaudhury, A.; Tiwari, B.; Kumar, S.; Kumari, R.; Bhat, K.V. Morphophysiological and biochemical response of mungbean (vigna radiata L.) varieties at different developmental stages under drought stress. Turkish J. Biol. 2019, 43, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, H.; Carter, T.E.; Purcell, L.C.; King, C.A.; Ries, L.L.; Chen, P.; Schapaugh, W.; Sinclair, T.R.; Boerma, H.R. Mapping of quantitative trait loci for canopy-wilting trait in soybean (Glycine max L. Merr). Theor. Appl. Genet. 2012, 125, 837–846. [Google Scholar] [CrossRef]
- Charlson, D.V.; Bhatnagar, S.; King, C.A.; Ray, J.D.; Sneller, C.H.; Carter, T.E.; Purcell, L.C. Polygenic inheritance of canopy wilting in soybean [Glycine max L. Merr.]. Theor. Appl. Genet. 2009, 119, 587–594. [Google Scholar] [CrossRef]
- Agbicodo, E.M.; Fatokun, C.A.; Muranaka, S.; Visser, R.G.F. Breeding drought tolerant cowpea: Constraints, accomplishments, and future prospects. Euphytica 2009, 167, 353–370. [Google Scholar] [CrossRef]
- Kumar, J.; Basu, P.S.; Srivastava, E.; Chaturvedi, S.K.; Nadarajan, N.; Kumar, S. Phenotyping of traits imparting drought tolerance in lentil. Crop Pasture Sci. 2012, 63, 547. [Google Scholar] [CrossRef]
- Upadhyaya, H.D.; Kashiwagi, J.; Varshney, R.K.; Gaur, P.M.; Saxena, K.B.; Krishnamurthy, L.; Gowda, C.L.L.; Pundir, R.P.S.; Chaturvedi, S.K.; Basu, P.S.; et al. Phenotyping chickpeas and pigeonpeas for adaptation to drought. Front. Physiol. 2012, 3, 179. [Google Scholar] [CrossRef]
- Fried, H.G.; Narayanan, S.; Fallen, B. Evaluation of soybean [Glycine max L. Merr.] genotypes for yield, water use efficiency, and root traits. PLoS ONE 2019, 14, e0212700. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.J.; Murphy, M.; Mutava, R.N.; Zhang, Z.; Nguyen, N.; Kim, Y.H.; Pathan, S.M.; Shannon, G.J.; Valliyodan, B.; Nguyen, H.T. Evaluation of high yielding soybean germplasm under water limitation. J. Integr. Plant Biol. 2016, 58, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Baldocchi, D.D.; Verma, S.B.; Rosenberg, N.J. Water use efficiency in a soybean field: influence of plant water stress. Agric. For. Meteorol. 1985, 34, 53–65. [Google Scholar] [CrossRef]
- Westgate, M.E.; Schussler, J.R.; Reicosky, D.C.; Brenner, M.L. Effect of water deficits on seed development in soybean. Plant Physiol. 1989, 91, 980–985. [Google Scholar] [CrossRef]
- Chen, Y.; Ghanem, M.E.; Siddique, K.H.M. Characterising root trait variability in chickpea (Cicer arietinum L.) germplasm. J. Exp. Bot. 2017, 68, 1987–1999. [Google Scholar] [CrossRef]
- Ramamoorthy, P.; Lakshmanan, K.; Upadhyaya, H.D.; Vadez, V.; Varshney, R.K. Shoot traits and their relevance in terminal drought tolerance of chickpea (Cicer arietinum L.). F. Crop. Res. 2016, 197, 10–27. [Google Scholar] [CrossRef]
- Serraj, R.; Chauhan, Y.S.; Bidinger, F.R.; Seetharama, N.; Nigam, S.N.; Saxena, N.P. Management of drought in icrisat cereal and legume mandate crops. In Water Productivity in Agriculture: Limits and Opportunities for Improvement; CAB International: Wallingford, UK, 2003; pp. 127–144. [Google Scholar]
- Singh, K.B.; Bejiga, G.; Saxena, M.C.; Singh, M. Transferability of chickpea selection indices from normal to drought-prone growing conditions in a mediterranean environment. J. Agron. Crop Sci. 1995, 175, 57–63. [Google Scholar] [CrossRef]
- Ghanbari, A.A.; Shakiba, M.R.; Toorchi, M.; Akbar, A. Morpho-physiological responses of common bean leaf to water deficit stress. Eur. J. Exp. Biol. 2013, 3, 487–492. [Google Scholar]
- Polania, J.; Rao, I.M.; Cajiao, C.; Grajales, M.; Rivera, M.; Velasquez, F.; Raatz, B.; Beebe, S.E. Shoot and root traits contribute to drought resistance in recombinant inbred lines of MD 23–24 × SEA 5 of common bean. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Beebe, S.E.; Rao, I.M.; Blair, M.W.; Acosta-Gallegos, J.A. Phenotyping common beans for adaptation to drought. Front. Physiol. 2013, 4, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, Y.S.; Wallace, D.H.; Johansen, C.; Singh, L. Genotype-by-environment interaction effect on yield and its physiological bases in short-duration pigeonpea. F. Crop. Res. 1998, 59, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Lopez, F.B.; Chauhan, Y.S.; Johansen, C. Effects of timing of drought stress on leaf area development and canopy light interception of short-duration pigeonpea. J. Agron. Crop Sci. 1997, 178, 1–7. [Google Scholar] [CrossRef]
- Hossain, M.; Hamid, A.; Khaliq, M. Evaluation of mungbean (Vigna Radiata L.) genotypes on the basis of photosynthesis and dry matter accumulation. J. Agric. Rural. Dev. 2010, 7, 1–8. [Google Scholar] [CrossRef]
- Khan, H.R.; Paull, J.G.; Siddique, K.H.M.; Stoddard, F.L. Faba bean breeding for drought-affected environments: A physiological and agronomic perspective. F. Crop. Res. 2010, 115, 279–286. [Google Scholar] [CrossRef]
- Belachew, K.Y.; Nagel, K.A.; Fiorani, F.; Stoddard, F.L. Diversity in root growth responses to moisture deficit in young faba bean (Vicia faba L.) plants. PeerJ 2018, 6, e4401. [Google Scholar] [CrossRef] [PubMed]
- Siddique, K.H.M.; Johansen, C.; Alghamdi, S.S. Innovations in agronomy for food legumes. A review. Agron. Sustain. Dev. 2012, 32, 45–64. [Google Scholar] [CrossRef]
- Jha, S.; Sehgal, V.K.; Rao, Y.V.S. Effect of sowing direction and crop geometry on water use efficiency and productivity of Indian mustard (Brassica juncea. L.) in semi arid region of India. J. Oilseed Brassica 2015, 6, 257–264. [Google Scholar]
- Hamza, M.A.; Anderson, W. Response of soil properties and grain yield to deep ripping and gypsum application in a compacted loamy sand soil contrasted with a sandy clay loam in Western Australia. Aust. J. Agric. Res. 2003, 54, 273–282. [Google Scholar] [CrossRef]
- Mupangwa, W.; Maize, I.; Twomlow, S.; Walker, S.; Africa, S.; Hove, L. Effect of minimum tillage and mulching on maize (Zea mays L.) yield and water content of clayey and sandy soils. Phys. Chem. Earth 2007, 32, 1127–1134. [Google Scholar] [CrossRef]
- Johnsona, Y.K.; Fredrick, O.A.; Josiah, M.K.; Sijali, I.V. Effects of tillage practices on water use efficiency and yield of different effects of tillage practices on water use efficiency and yield of different drought tolerant common bean varieties in machakos county, Eastern Kenya. Am. Sci. Res. J. Eng. Technol. Sci. 2018, 40, 217–234. [Google Scholar] [CrossRef]
- Kumar, A.; Bernier, J.; Verulkar, S.; Lafitte, H.R.; Atlin, G.N. Breeding for drought tolerance: Direct selection for yield, response to selection and use of drought-tolerant donors in upland and lowland-adapted populations. F. Crop. Res. 2008, 107, 221–231. [Google Scholar] [CrossRef]
- Turner, N.C. Agronomic options for improving rainfall-use efficiency of crops in dryland farming systems. J. Exp. Bot. 2004, 55, 2413–2425. [Google Scholar] [CrossRef] [Green Version]
- OConnell, M.G.; Leary, G.J.O.; Whitfield, D.M.; Connor, D.J. Interception of photosynthetically active radiation and radiation-use efficiency of wheat, field pea and mustard in a semi-arid environment. F. Crop. Res. 2004, 85, 111–124. [Google Scholar] [CrossRef]
- Matsuo, N.; Yamada, T.; Takada, Y.; Fukami, K.; Hajika, M. Effect of plant density on growth and yield of new soybean genotypes grown under early planting condition in southwestern Japan. Plant Prod. Sci. 2018, 21, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.B.; Yang, G.M.; Sun, S.J.; Chen, Y.H. Plant and row spacing effects on soil water and yield of rainfed summer soybean in the northern China. Plant Soil Environ. 2010, 56, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Agajie, M. Effect of spacing on yield components and yield of chickpea (Cicer arietinum L.) at Assosa, Western Ethiopia. Agric. For. Fish. 2018, 7, 39–51. [Google Scholar] [CrossRef]
- Hansel, F.D.; Amado, T.J.C.; Diaz, D.A.R.; Rosso, L.H.M.; Nicoloso, F.T.; Schorr, M. Phosphorus fertilizer placement and tillage affect soybean root growth and drought tolerance. Agron. J. 2017, 109, 2936–2944. [Google Scholar] [CrossRef]
- Jin, J.; Wang, G.; Liu, X.; Pan, X.; Herbert, S.J.; Tang, C. Interaction between phosphorus nutrition and drought on grain yield, and assimilation of phosphorus and nitrogen in two soybean cultivars differing in protein concentration in grains. J. Plant Nutr. 2006, 29, 1433–1449. [Google Scholar] [CrossRef]
- Sangakkara, U.R.; Frehner, M.; Nosberger, J. Effect of soil moisture and potassium fertilizer on shoot water potential, photosynthesis and partitioning of carbon in mungbean and cowpea. J. Agron. Crop Sci. 1999, 185, 201–207. [Google Scholar] [CrossRef]
- Palta, J.A.; Kumari, S.; Spring, C.; Turner, N.C. Foliar nitrogen applications increase the seed yield and protein content in chickpea (Cicer arietinum L.) subject to terminal drought. Aust. J. Agr. Res. 2005, 56, 105–112. [Google Scholar] [CrossRef]
- Hati, K.M.; Mandal, K.G.; Misra, A.K.; Ghosh, P.K.; Bandyopadhyay, K.K. Effect of inorganic fertilizer and farmyard manure on soil physical properties, root distribution, and water-use efficiency of soybean in Vertisols of central India. Bioresour. Technol. 2006, 97, 2182–2188. [Google Scholar] [CrossRef]
- Bandyopadhyay, K.K.; Misra, A.K.; Ghosh, P.K.; Hati, K.M. Effect of integrated use of farmyard manure and chemical fertilizers on soil physical properties and productivity of soybean. Soil Tillage Res. 2010, 110, 115–125. [Google Scholar] [CrossRef]
- Leport, L.; Turner, N.C.; Davies, S.L.; Siddique, K.H.M. Variation in pod production and abortion among chickpea cultivars under terminal drought. Eur. J. Agron. 2006, 24, 236–246. [Google Scholar] [CrossRef]
- Mohammadi, A.; Habibi, D.; Rohami, M.; Mafakheri, S. Effect of drought stress on antioxidant enzymes activity of some chickpea cultivars. Am-Eurasian J. Agric. Env. Sci. 2011, 11, 782–785. [Google Scholar]
- Grams, T.E.E.; Koziolek, C.; Lautner, S.; Matyssek, R. Distinct roles of electric and hydraulic signals on the reaction of leaf gas exchange upon re-irrigation in Zea mays L. Plant Cell Environ. 2007, 30, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Inanaga, S.; Araki, H.; An, P.; Morita, S.; Luxová, M.; Lux, A. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plant. 2005, 123, 459–466. [Google Scholar] [CrossRef]
- Xu, C.X.; Ma, Y.P.; Liu, Y.L. Effects of silicon (Si) on growth, quality and ionic homeostasis of aloe under salt stress. South Afr. J. Bot. 2015, 98, 26–36. [Google Scholar] [CrossRef]
- Mali, M.; Aery, N.C. Silicon effects on nodule growth, dry-matter production, and mineral nutrition of cowpea (Vigna unguiculata L.). J. Plant Nutr. Soil Sci. 2008, 171, 835–840. [Google Scholar] [CrossRef]
- Kurdali, F.; Al-chammaa, M.; Mouasess, A. Growth and nitrogen fixation in silicon and/or potassium fed chickpeas grown under drought and well watered conditions. J. Stress Physiol. Biochem. 2016, 9, 385–406. [Google Scholar]
- Kurdali, F.; Al-Chammaa, M.; Al-Ain, F. Growth and N2 fixation in saline and/or water stressed Sesbania aculeata plants in response to silicon application. Silicon 2019, 11, 781–788. [Google Scholar] [CrossRef]
- Shaban, M.; Lak, M.; Hamidvand, Y.; Nabaty, E.; Khodaei, F. Response of chickpea (Cicer arietinum L.) cultivars to integrated application of Zinc nutrient with water stress. Int. J. Agric. Crop Sci. 2012, 4, 1074–1082. [Google Scholar]
- Thalooth, A.T.; Tawfik, M.M.; Mohamed, H.M. A Comparative study on the effect of foliar application of zinc, potassium and magnesium on growth, yield and some chemical constituents of mungbean plants grown under water stress conditions. World J. Agric. Sci. 2006, 2, 37–46. [Google Scholar]
- Yadavi, A.; Movahhedi-dehnavi, M.; Balouchi, H. Effect of micronutrients foliar application on grain qualitative characteristics and some physiological traits of bean (Phaseolus vulgaris L.) under drought stress. Indian J. Fundam. Appl. Life Sci. 2014, 4, 124–131. [Google Scholar]
- Bellaloui, N.; Hu, Y.; Mengistu, A.; Kassem, M.A.; Abel, C.A. Effects of foliar boron application on seed composition, cell wall boron, and seed δ15 N and δ13 C isotopes in water-stressed soybean plants. Front. Plant Sci. 2013, 4, 270. [Google Scholar] [CrossRef] [PubMed]
- Bellaloui, N.; Mengistu, A. Effects of boron nutrition and water stress on nitrogen fixation, seed δ 15 N and δ 13 C dynamics, and seed composition in soybean cultivars differing in maturities. Sci. World J. 2015, 2015, 407872. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Babar, A. Metabolic and physiological changes induced by plant growth regulators and plant growth promoting rhizobacteria and their impact on drought tolerance in Cicer arietinum L. PLoS ONE 2019, 13, e0213040. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Cheng, Z.; Czarny, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur. J. Plant Pathal. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Ashraf, M.; Nadeem, S.M.; Zahir, Z.A.; Naveed, M. Microbial ACC-deaminase: Prospects and applications for inducing salt tolerance in plants microbial. Crit. Rev. Plant Sci. 2010, 29, 360–393. [Google Scholar] [CrossRef]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W.; Job, D. Drought-tolerant plant growth-promoting rhizobacteria associated with Foxtail Millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2018, 8, 2580. [Google Scholar] [CrossRef] [PubMed]
- Hayat, R.; Ali, S.; Amara, U. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Belimov, A.A.; Dodd, I.C.; Hontzeas, N.; Theobald, J.C.; Safronova, V.I.; Davies, W.J. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol. 2009, 181, 413–423. [Google Scholar] [CrossRef]
- Long, H.H.; Schmidt, D.D.; Baldwin, I.T. Native bacterial endophytes promote host growth in a species-specific manner; phytohormone manipulations do not result in common growth responses. PLoS ONE 2008, 3, e2702. [Google Scholar] [CrossRef]
- Barka, E.A.; Nowak, J.; Cle, C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, burkholderia phytofirmans strain PsJN. Appl. Environ. Microbiol. 2006, 72, 7246–7252. [Google Scholar] [CrossRef]
- Sziderics, A.H.; Rasche, F.; Trognitz, F.; Sessitsch, A.; Wilhelm, E. Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Can. J. Microbiol. 2007, 53, 1195–1202. [Google Scholar] [CrossRef]
- Bashan, Y.; Alcaraz, L. Responses of soybean and cowpea root membranes to inoculation with Azospirillum brasilense. Symbiosis 1992, 13, 217–228. [Google Scholar]
- Nadeem, M.S.; Ahmad, M.; Ahmad, Z.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Smith, S.E.; Facelli, E.; Pope, S.; Smith, F.A. Plant performance in stressful environments: Interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 2010, 326, 3–20. [Google Scholar] [CrossRef]
- Porcel, R.; Ruiz-lozano, J.M. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J. Exp. Bot. 2004, 55, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh, Y.; Evazi, A.R.; Abedi, M. Alleviation drought stress of mungbean (Vigna radiata L.) plants by using arbuscular mycorrhizal fungi. Int. J. Agric. Sci. Nat. Resour. 2014, 1, 1–6. [Google Scholar]
- Porcel, R.; Barea, J.M.; Ruiz-lozano, J.M.; Ruiz-lozano, J.M. Antioxidant activities in mycorrhizal soybean plants under drought stress and their possible relationship to the process of nodule senescence. New Phytol. 2003, 157, 135–143. [Google Scholar] [CrossRef]
- Sohrabi, Y.; Heidari, G.; Weisany, W.; Golezani, K.G.; Mohammadi, K. Some physiological responses of chickpea cultivars to arbuscular mycorrhiza under drought stress. Russ. J. Plant Physiol. 2012, 59, 708–716. [Google Scholar] [CrossRef]
- Gaur, A.; Adholeya, A. Arbuscular-mycorrhizal inoculation of five tropical fodder crops and inoculum production in marginal soil amended with organic matter. Biol. Fertil. Soils 2002, 35, 214–218. [Google Scholar] [CrossRef]
- Figueiredo, V.B.; Burity, A.; Martı, C.R.; Chanway, C.P. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 2008, 40, 182–188. [Google Scholar] [CrossRef]
- Leggett, M.; Diaz-Zorita, M.; Koivunen, M.; Bowman, R.; Pesek, R.; Stevenson, C.; Leister, T. Soybean response to inoculation with in the United States and Argentina. Agron. J. 2017, 109, 1031. [Google Scholar] [CrossRef]
- Ulzen, J.; Abaidoo, R.C.; Masso, C.; Mensah, N.E.; AbdelGadir, A.H. Bradyrhizobium inoculants enhance grain yields of soybean and cowpea in Northern Ghana. Front. Plant Sci. 2016, 7, 1770. [Google Scholar] [CrossRef] [PubMed]
- Aliasgharzad, N.; Neyshabouri, M.R.; Salimi, G. Effects of arbuscular mycorrhizal fungi and Bradyrhizobium japonicum on drought stress of soybean. Biol. Bratislava 2006, 61, 324–328. [Google Scholar] [CrossRef]
- Cattelan, A.J.; Hartel, P.G.; Fuhrmann, J.J. Screening for plant growth-promoting rhizobacteria to promote early soybean growth. Soil Sci. Soc. Am. J. 1996, 63, 1670–1680. [Google Scholar] [CrossRef]
- Li, D.M.; Alexander, M. Co-inoculation with antibiotic-producing bacteria to increase colonization and nodulation by rhizobia. Plant Soil 1988, 108, 211–219. [Google Scholar] [CrossRef]
- Stancheva, I.; Geneva, M.; Hristozkova, M.; Sichanova, M.; Donkova, R.; Petkova, G.; Djonova, E. Response of Vigna Unguiculata grown under different soil moisture regimes to the dual inoculation with nitrogen-fixing Bacteria and Arbuscular Mycorrhizal Fungi. Commun. Soil Sci. Plant Anal. 2017, 48, 1378–1386. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-lozano, J.M.; Aroca, R. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol. 2006, 173, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Augé, R.M. Arbuscular mycorrhizae and soil/plant water relations. Can. J. Soil Sci. 2004, 84, 373–381. [Google Scholar] [CrossRef]
- German, M.A.; Burdman, S.; Okon, Y. Effects of Azospirillum brasilense on root morphology of common bean (Phaseolus vulgaris L.) under different water regimes. Biol. Fertil. Soils 2000, 32, 259–264. [Google Scholar] [CrossRef]
- Srinivasan, M.; Petersen, J.; Holl, B. Lnfluence of indoleacetic-acid-producing Bacillus isolates on the nodulation of Phaseolus vulgaris by Rhizobium etli under gnotobiotic conditions. Can. J. Microbiol. 1996, 42, 1006–1014. [Google Scholar] [CrossRef]
- Ferreira, L.d.V.M.; Carvalho, F.D.; Andrade, J.F.C.; Moreira, F.M.D.S. Growth promotion of common bean and genetic diversity of bacteria from Amazon pastureland. Sci. Agric. 2018, 75, 461–469. [Google Scholar] [CrossRef]
- Habibzadeh, Y. Response of mung bean plants to arbuscular mycorrhiza and phosphorus in drought stress. Int. J. Innov. Appl. Stud. 2014, 6, 2028–9324. [Google Scholar]
- Mayak, S.; Tirosh, T.; Glick, B.R. Effect of wild-type and mutant plant growth-promoting rhizobacteria on the rooting of mung bean cuttings. J. Plant Growth Regul. 1999, 18, 49–53. [Google Scholar] [CrossRef]
- Chanway, C.P.; Hynes, R.K.; Nelson, L.M. Plant growth-promoting rhizobacteria: effects on growth and nitrogen fixation of lentil (Lens esculenta Moench) and pea (Pisum sativum L.). Soil Biol. Biochem. 1989, 21, 511–517. [Google Scholar] [CrossRef]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci. Rep. 2018, 8, 3560. [Google Scholar] [CrossRef] [PubMed]
- Frahm, M.A.; Rosas, J.C.; Mayek-Pérez, N.; López-Salinas, E.; Acosta-Gallegos, J.A.; Kelly, J.D. Breeding beans for resistance to terminal drought in the lowland tropics. Euphytica 2004, 136, 223–232. [Google Scholar] [CrossRef]
- Beebe, S.E.; Rao, I.M.; Cajiao, C.; Grajales, M. Selection for drought resistance in common bean also improves yield in phosphorus limited and favorable environments. Crop Sci. 2008, 48, 582–592. [Google Scholar] [CrossRef]
- Torres, A.M.; Avila, C.M.; Gutierrez, N.; Palomino, C.; Moreno, M.T.; Cubero, J.I. Marker-assisted selection in faba bean (Vicia faba L.). F. Crop. Res. 2010, 115, 243–252. [Google Scholar] [CrossRef]
- Mir, R.R.; Zaman-Allah, M.; Sreenivasulu, N.; Trethowan, R.; Varshney, R.K. Integrated genomics, physiology and breeding approaches for improving drought tolerance in crops. Theor. Appl. Genet. 2012, 125, 625–645. [Google Scholar] [CrossRef] [Green Version]
- Duc, G.; Agrama, H.; Bao, S.; Berger, J.; Bourion, V.; De Ron, A.M.; Gowda, C.L.L.; Mikic, A.; Millot, D.; Singh, K.B.; et al. Breeding annual grain legumes for sustainable agriculture: New methods to approach complex traits and target new cultivar ideotypes. CRC. Crit. Rev. Plant Sci. 2015, 34, 381–411. [Google Scholar] [CrossRef]
- Montes, J.M.; Melchinger, E.A.; Reif, J.C. Novel throughput phenotyping platforms in plant genetic studies. Trends Plant Sci. 2007, 12, 433–436. [Google Scholar] [CrossRef]
- Chapman, S.C. Use of crop models to understand genotype by environment interactions for drought in real-world and simulated plant breeding trials. Euphytica 2008, 161, 195–208. [Google Scholar] [CrossRef]
- Martynenko, A.; Shotton, K.; Astatkie, T.; Petrash, G.; Fowler, C.; Neily, W.; Critchley, A.T. Thermal imaging of soybean response to drought stress: the effect of Ascophyllum nodosum seaweed extract. Springerplus 2016, 5, 1393. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Tian, F.; Zhang, L.; Li, S.; Du, T.; Huang, M.; Yuan, Y. Estimating crop transpiration of soybean under different irrigation treatments using thermal infrared remote sensing imagery. Agronomy 2018, 9, 8. [Google Scholar] [CrossRef]
- Muchero, W.; Roberts, P.A.; Diop, N.N.; Drabo, I.; Cisse, N.; Close, T.J.; Muranaka, S.; Boukar, O.; Ehlers, J.D. Genetic architecture of delayed senescence, biomass, and grain yield under drought stress in cowpea. PLoS ONE 2013, 8, e70041. [Google Scholar] [CrossRef]
- Amede, T.; Kittlitz, E.V.; Schubert, S. Differential drought responses of faba bean (Vicia faba L.) inbred lines. J. Agron. Crop Sci. 1999, 183, 35–45. [Google Scholar] [CrossRef]
- Ribeiro, T.; da Silva, D.A.; Esteves, J.A.d.F.; Azevedo, C.V.G.; Gonçalves, J.G.R.; Carbonell, S.A.M.; Chiorato, A.F. Evaluation of common bean genotypes for drought tolerance. Bragantia 2019, 78, 1–11. [Google Scholar] [CrossRef]
- Krishnamurthy, L.; Kashiwagi, J.; Tobita, S.; Ito, O.; Upadhyaya, H.D.; Gowda, C.L.L.; Gaur, P.M.; Sheshshayee, M.S.; Singh, S.; Vadez, V.; et al. Variation in carbon isotope discrimination and its relationship with harvest index in the reference collection of chickpea germplasm. Funct. Plant Biol. 2013, 40, 1350. [Google Scholar] [CrossRef]
- Singh, M.; Rani, S.; Malhotra, N.; Katna, G.; Sarker, A. Transgressive segregations for agronomic improvement using interspecific crosses between c. Arietinum L. × c. Reticulatum Ladiz. And c. Arietinum L. × c. Echinospermum davis species. PLoS ONE 2018, 13, e0203082. [Google Scholar] [CrossRef]
- Parsons, L.R.; Howe, T.K. Effects of water stress on the water relations of Phaseolus vulgaris and the drought resistant Phaseolus acutifolius. Physiol. Plant. 1984, 60, 197–202. [Google Scholar] [CrossRef]
- Basu, P.S.; Berger, J.D.; Turner, N.C.; Chaturvedi, S.K.; Ali, M.; Siddique, K.H.M. Osmotic adjustment of chickpea (Cicer arietinum L.) is not associated with changes in carbohydrate composition or leaf gas exchange under drought. Ann. Appl. Biol. 2007, 150, 217–225. [Google Scholar] [CrossRef]
- Kashiwagi, J.; Krishnamurthy, L.; Upadhyaya, H.D.; Krishna, H.; Chandra, S.; Vadez, V.; Serraj, R. Genetic variability of drought-avoidance root traits in the mini-core germplasm collection of chickpea (Cicer arietinum L.). Euphytica 2005, 146, 213–222. [Google Scholar] [CrossRef]
- Hamwieh, A.; Imtiaz, M.; Maize, I.; Malhotra, R.S. Multi-environment QTL analyses for drought-related traits in a recombinant inbred population of chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2013, 126, 1025–1038. [Google Scholar] [CrossRef]
- Radhika, P.; Gowda, S.J.M.; Kadoo, N.Y.; Mhase, L.B.; Jamadagni, B.M.; Sainani, M.N.; Chandra, S.; Gupta, V.S. Development of an integrated intraspecific map of chickpea (Cicer arietinum L.) using two recombinant inbred line populations. Theor. Appl. Genet. 2007, 115, 209–216. [Google Scholar] [CrossRef]
- Muchero, W.; Je, V.; Close, T.J.; Roberts, P.A. Mapping QTL for drought stress-induced premature senescence and maturity in cowpea (Vigna unguiculata L.). Theor. Appl. Genet. 2009, 118, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Pipolo, A.E.; Boerma, H.R.; Sinclair, T.R. Identification of QTLs associated with limited leaf hydraulic conductance in soybean. Euphytica 2011, 186, 679–686. [Google Scholar] [CrossRef]
- Mian, M.A.R.; Bailey, M.A.; Ashley, D.A.; Wells, R.; Carter, T.E.; Parrott, W.A.; Boerma, H.R. Molecular markers associated with water use efficiency and leaf ash in soybean. Crop Sci. 1996, 36, 1252–1257. [Google Scholar] [CrossRef]
- Manavalan, L.P.; Guttikonda, S.K.; Tran, L.P.; Nguyen, H.T. Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 2009, 50, 1260–1276. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haleem, H.; Lee, G.J.; Boerma, R.H. Identification of QTL for increased fibrous roots in soybean. Theor. Appl. Genet. 2011, 122, 935–946. [Google Scholar] [CrossRef]
- Khazaei, H.; Sullivan, D.M.O.; Sillanpää, M.J.; Stoddard, F.L. Use of synteny to identify candidate genes underlying QTL controlling stomatal traits in faba bean (Vicia faba L.). Theor. Appl. Genet. 2014, 127, 2371–2385. [Google Scholar] [CrossRef]
- Mukeshimana, G.; Butare, L.; Cregan, P.B.; Blair, M.W.; Kelly, J.D. Quantitative trait loci associated with drought tolerance in common bean. Crop Sci. 2014, 54, 923–938. [Google Scholar] [CrossRef]
- Briñez, B.; Morini, J.; Cardoso, K.; Rosa, J.S.; Bassi, D.; Gonçalves, G.R.; Almeida, C.; Fausto, J.; Paulino, D.C.; Blair, M.W.; et al. Mapping QTLs for drought tolerance in a SEA 5 × AND 277 common bean cross with SSRs and SNP markers. Genet. Mol. Biol. 2017, 823, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Kishor, P.B.K.; Hong, Z.; Miao, C.-H.; Hu, C.-A.A.; Verma, D.P.S. Overexpression of A1-Pyrroline-5-Carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol. 1995, 108, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar-Mathur, P.; Vadez, V.; Jyostna Devi, M.; Lavanya, M.; Vani, G.; Sharma, K.K. Genetic engineering of chickpea (Cicer arietinum L.) with the P5CSF129A gene for osmoregulation with implications on drought tolerance. Mol. Breed. 2009, 23, 591–606. [Google Scholar] [CrossRef]
- Anbazhagan, K.; Bhatnagar-Mathur, P.; Vadez, V.; Dumbala, S.R.; Kishor, P.K.; Sharma, K.K. DREB1A overexpression in transgenic chickpea alters key traits influencing plant water budget across water regimes. Plant Cell Rep. 2014, 34, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, J.; Zhang, J.; Hao, L.; Hua, J.; Duan, L.; Zhang, M.; Li, Z. Expression of an Arabidopsis molybdenum cofactor sulphurase gene in soybean enhances drought tolerance and increases yield under field conditions. Plant Biotechnol. J. 2013, 11, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, S.; Kobayashi, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A Stress-inducible gene for 9-cis-epoxycarotenoid dioxygenase involved in abscisic acid biosynthesis under water stress in drought-tolerant cowpea. Plant Physiol. 2002, 123, 553–562. [Google Scholar] [CrossRef]
- An, J.; Cheng, C.; Hu, Z.; Chen, H.; Cai, W.; Yu, B. The Panax ginseng PgTIP1 gene confers enhanced salt and drought tolerance to transgenic soybean plants by maintaining homeostasis of water, salt ions and ROS. Environ. Exp. Bot. 2018, 155, 45–55. [Google Scholar] [CrossRef]
- Savitri, E.S.; Fauziah, S.M. Characterization of drought tolerance of GmDREB2 soybean mutants (Glycine max L.) by ethyl methane sulfonate induction. AIP Conf. Proc. 2018, 2019. [Google Scholar] [CrossRef]
- Li, D.H.; Li, W.; Li, H.Y.; Guo, J.J.; Chen, F.J. The soybean GmRACK1 gene plays a role in drought tolerance at vegetative stages. Russ. J. Plant Physiol. 2018, 65, 541–552. [Google Scholar] [CrossRef]
- Kim, H.J.; Cho, H.S.; Pak, J.H.; Kwon, T.; Lee, J.; Kim, D.; Lee, D.H.; Kim, C.; Chung, Y. Molecules and cells confirmation of drought tolerance of ectopically expressed AtABF3 gene in soybean. Mol. Cells 2018, 41, 413–422. [Google Scholar]
- Li, Y.; Chen, Q.; Nan, H.; Li, X.; Lu, S.; Zhao, X.; Liu, B.; Guo, C.; Kong, F.; Cao, D. Overexpression of GmFDL19 enhances tolerance to drought and salt stresses in soybean. PLoS ONE 2017, 12, e0179554. [Google Scholar] [CrossRef]
- Chen, Y.; Chi, Y.; Meng, Q.; Wang, X.; Yu, D. GmSK1, an SKP1 homologue in soybean, is involved in the tolerance to salt and drought. Plant Physiol. Biochem. 2018, 127, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar-Mathur, P.; Devi, M.J.; Reddy, D.S.; Lavanya, M.; Vadez, V.; Serraj, R.; Yamaguchi-Shinozaki, K.; Sharma, K.K. Stress-inducible expression of At DREB1A in transgenic peanut (Arachis hypogaea L.) increases transpiration efficiency under water-limiting conditions. Plant Cell Rep. 2007, 26, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Chen, Q.S.; Xin, D.W.; Qi, Z.M.; Zhang, C.; Li, S.N.; Jin, Y.; Li, M.; Mei, H.Y.; Su, A.Y.; et al. Overexpression of GmBIN2, a soybean glycogen synthase kinase 3 gene, enhances tolerance to salt and drought in transgenic Arabidopsis and soybean hairy roots. J. Integr. Agric. 2018, 17, 1959–1971. [Google Scholar] [CrossRef]
- Yoo, J.H.; Park, C.Y.; Cheol, J.; Do Heo, W.; Cheong, M.S.; Park, H.C.; Kim, M.C.; Moon, B.C.; Choi, M.S.; Kang, Y.H.; et al. Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in Arabidopsis. J. Biol. Chem. 2005, 280, 3697–3706. [Google Scholar] [CrossRef]
- Guenther, J.F.; Chanmanivone, N.; Galetovic, M.P.; Wallace, I.S.; Cobb, J.A.; Roberts, D.M. Phosphorylation of soybean nodulin 26 on serine 262 enhances water permeability and is regulated developmentally and by osmotic signals. Plant Cell 2003, 15, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.; Karakaya, H.C.; Knap, H.T. Molecular characterization of two soybean homologs of Arabidopsis thaliana CLAVATA1 from the wild type and fasciation. Biochim. Biophys. Acta 2000, 1491, 333–340. [Google Scholar] [CrossRef]
- Azeem, F.; Bilal, A.; Rana, M.A.; Muhammad, A.A.; Habibullah, N.; Sabir, H.; Sumaira, R.; Hamid, M.; Usama, A.; Muhammad, A. Drought affects aquaporins gene expression in important pulse legume chickpea (Cicer arietinum L.). Pak. J. Bot. 2019, 51, 81–88. [Google Scholar] [CrossRef]
- Nayak, S.N.; Balaji, J.; Upadhyaya, H.D.; Hash, C.T.; Kishor, P.B.K.; Blair, M.W.; Baum, M.; Chattopadhyay, D.; Marı, L.; Mcnally, K.; et al. Plant science isolation and sequence analysis of DREB2A homologues in three cereal and two legume species. Plant Sci. 2009, 177, 460–467. [Google Scholar] [CrossRef]
- Hiremath, P.J.; Farmer, A.; Cannon, S.B.; Woodward, J.; Kudapa, H.; Tuteja, R.; Kumar, A.; Bhanuprakash, A.; Mulaosmanovic, B.; Gujaria, N.; et al. Large-scale transcriptome analysis in chickpea (Cicer arietinum L.), an orphan legume crop of the semi-arid tropics of Asia and Africa. Plant Biotechnol. J. 2011, 9, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Deokar, A.A.; Kondawar, V.; Jain, P.K.; Karuppayil, S.M.; Raju, N.L.; Vadez, V. Comparative analysis of expressed sequence tags (ESTs) between drought-tolerant and -susceptible genotypes of chickpea under terminal drought stress. BMC Plant Biol. 2011, 11, 70. [Google Scholar] [CrossRef]
- Muchero, W.; Je, V.; Roberts, P.A. Restriction site polymorphism-based candidate gene mapping for seedling drought tolerance in cowpea [Vigna unguiculata (L.) Walp.]. Theor. Appl. Genet. 2010, 120, 509–518. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, J.; Li, X.; Wang, S.; Wu, J. Salt and drought stress and ABA responses related to bZIP genes from V. radiata and V. angularis. Gene 2018, 651, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Baloda, A.; Madanpotra, S.; Aiwal, P.K. Transformation of mungbean plants for salt and drought tolerance by introducing a gene for an osmoprotectant glycine betaine. J. Plant Stress Physiol. 2017, 3, 5. [Google Scholar] [CrossRef]
- Srivastava, R.; Kumar, S.; Kobayashi, Y.; Kusunoki, K.; Tripathi, P.; Kobayashi, Y.; Koyama, H.; Sahoo, L. Comparative genome-wide analysis of WRKY transcription factors in two Asian legume crops: Adzuki bean and Mung bean. Sci. Rep. 2018, 8, 16971. [Google Scholar] [CrossRef] [Green Version]
- Cortés, A.J.; This, D.; Carolina, C.; Madriñán, S.; Blair, M.W. Nucleotide diversity patterns at the drought-related DREB2 encoding genes in wild and cultivated common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2012, 125, 1069–1085. [Google Scholar] [CrossRef]
- Barrera-Figueroa, B.E.; Peña-Castro, J.M.; Acosta-Gallegos, J.A.; Ruiz-Medrano, R.; Beatriz, X.-C. Isolation of dehydration-responsive genes in a drought tolerant common bean cultivar and expression of a group 3 late embryogenesis abundant mRNA in tolerant and susceptible bean cultivars. Funct. Plant Biol. 2007, 34, 368–381. [Google Scholar] [CrossRef]
- Saxena, K.B.; Singh, G.; Gupta, H.S.; Mahajan, V.; Kumar, R.V.; Singh, B.; Vales, M.I. Enhancing the livelihoods of Uttarakhand farmers by introducing pigeonpea cultivation in hilly areas. J. Food Legum. 2011, 24, 128–132. [Google Scholar]
- Yang, S.; Pang, E.W.; Ash, E.G.; Huttner, E.; Zong, E.X.; Kilian, E.A. Low level of genetic diversity in cultivated Pigeonpea compared to its wild relatives is revealed by diversity arrays technology. Theor. Appl. Genet. 2006, 113, 585–595. [Google Scholar] [CrossRef]
- Cui, X.H.; Hao, F.S.; Chen, H.; Chen, J.; Wang, X.C. Expression of the Vicia faba VfPIP1 gene in Arabidopsis thaliana plants improves their drought resistance. J. Plant Res. 2008, 121, 207–214. [Google Scholar] [CrossRef]
- Zheng, G.; Fan, C.; Di, S.; Wang, X.; Xiang, C.; Pang, Y. Over-expression of Arabidopsis EDT1 gene confers drought tolerance in alfalfa (Medicago sativa L.). Front. Plant Sci. 2017, 8, 2125. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Feyissa, B.A.; Amyot, L.; Aung, B.; Hannoufa, A. MicroRNA156 improves drought stress tolerance in alfalfa (Medicago sativa L.) by silencing SPL13. Plant Sci. 2017, 258, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Duan, Z.; Zhang, D.; Zhang, J.; Di, H.; Wu, F.; Wang, Y. Co-transforming bar and CsLEA enhanced tolerance to drought and salt stress in transgenic alfalfa (Medicago sativa L.). Biochem. Biophys. Res. Commun. 2016, 472, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Cai, H.; Ji, W.; Luo, X.; Wang, Z.; Wu, J.; Wang, X.; Cui, L.; Wang, Y.; Zhu, Y.; et al. Overexpression of GsZFP1 enhances salt and drought tolerance in transgenic alfalfa (Medicago sativa L.). Plant Physiol. Biochem. 2013, 71, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Z.; Ke, Q.; Ji, C.Y.; Jeong, J.C.; Lee, H.S.; Lim, Y.P.; Xu, B.; Deng, X.P.; Kwak, S.S. Overexpression of codA gene confers enhanced tolerance to abiotic stresses in alfalfa. Plant Physiol. Biochem. 2014, 85, 31–40. [Google Scholar] [CrossRef]
- Cabello, J.V.; Giacomelli, J.I.; Gómez, M.C.; Chan, R.L. The sunflower transcription factor HaHB11 confers tolerance to water deficit and salinity to transgenic Arabidopsis and alfalfa plants. J. Biotechnol. 2017, 257, 35–46. [Google Scholar] [CrossRef]
- Bao, A.K.; Wang, S.M.; Wu, G.Q.; Xi, J.J.; Zhang, J.L.; Wang, C.M. Overexpression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.). Plant Sci. 2009, 176, 232–240. [Google Scholar] [CrossRef]
- Joshi, T.; Yan, Z.; Libault, M.; Jeong, D.; Park, S.; Green, P.J.; Sherrier, D.J.; Farmer, A.; May, G.; Meyers, B.C.; et al. Prediction of novel miRNAs and associated target genes in Glycine max L. BMC Bioinform. 2010, 11, S14. [Google Scholar] [CrossRef]
- Song, Q.; Liu, Y.; Hu, X.; Zhang, W.; Ma, B.; Chen, S.; Zhang, J. Identification of miRNAs and their target genes in developing soybean seeds by deep sequencing. BMC Plant Biol. 2011, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, S.; Liu, L.; Ma, Y.; Li, S.; Zu, W. Transcriptome profiling reveals PEG-simulated drought, heat and combined stress response mechanisms in soybean. Comput. Biol. Chem. 2018. [Google Scholar] [CrossRef]
- Prince, S.J.; Joshi, T.; Mutava, R.N.; Syed, N.; Vitor, M.d.S.J.; Patil, G.; Song, L.; Wang, J.J.; Lin, L.; Chen, W.; et al. Comparative analysis of the drought-responsive transcriptome in soybean lines contrasting for canopy wilting. Plant Sci. 2015, 240, 65–78. [Google Scholar] [CrossRef]
- De Domenico, S.; Bonsegna, S.; Horres, R.; Pastor, V.; Taurino, M.; Poltronieri, P.; Imtiaz, M.; Kahl, G.; Flors, V.; Winter, P.; et al. Transcriptomic analysis of oxylipin biosynthesis genes and chemical profiling reveal an early induction of jasmonates in chickpea roots under drought stress. Plant Physiol. Biochem. 2012, 61, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Strozycki, P.M.; Szczurek, A.; Lotocka, B.; Figlerowicz, M.; Legocki, A.B. Ferritins and nodulation in Lupinus luteus: Iron management in indeterminate type nodules. J. Exp. Bot. 2007, 58, 3145–3153. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Valliyodan, B.; Zhang, J.; Lenoble, M.E.; Yu, O.; Rogers, E.E.; Nguyen, H.T.; Sharp, R.E. Regulation of growth response to water stress in the soybean primary root. I. Proteomic analysis reveals region-specific regulation of phenylpropanoid metabolism and control of free iron in the elongation zone. Plant Cell Environ. 2010, 33, 223–243. [Google Scholar] [CrossRef]
- Varshney, R.K.; Dubey, A. Novel genomic tools and modern genetic and breeding approaches for crop improvement. J. Plant Biochem. Biotechnol. 2009, 18, 127–138. [Google Scholar] [CrossRef]
- Pandey, A.; Chakraborty, S.; Datta, A.; Chakraborty, N. Proteomics approach to identify dehydration responsive nuclear proteins from chickpea (Cicer arietinum L.). Mol. Cell. Proteomics 2008, 7, 88–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Q.; Li, D.; Yang, X.; Li, D. Multifunctional roles of plant dehydrins in response to environmental stresses. Front. Plant Sci. 2017, 8, 2015–2018. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Rushton, P.; Rohila, J. Metabolomic profiling of soybeans (Glycine max L.) reveals the importance of sugar and nitrogen metabolism under drought and heat stress. Plants 2017, 6, 21. [Google Scholar] [CrossRef]
- Sanchez-Leon, S.; Gil-humanes, J.; Ozuna, C.V.; Sousa, C.; Voytas, D.F.; Barro, F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018, 16, 902–910. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, R.; Huang, G.; Li, Y.; Melaku, G.; Zhang, S.; Chen, H.; Zhao, Y.; Zhang, J.; Zhang, Y.; et al. Developing superior alleles of yield genes in rice by artificial mutagenesis using the CRISPR/Cas9 system. Crop J. 2018, 6, 475–481. [Google Scholar] [CrossRef]
- Lawrenson, T.; Shorinola, O.; Stacey, N.; Li, C.; Østergaard, L.; Patron, N.; Uauy, C.; Harwood, W. Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease. Genome Biol. 2015, 16, 258. [Google Scholar] [CrossRef]
- Kapusi, E.; Corcuera-Gómez, M.; Melnik, S.; Stoger, E. Heritable genomic fragment deletions and small indels in the putative ENGase gene induced by CRISPR/Cas9 in barley. Front. Plant Sci. 2017, 8, 540. [Google Scholar] [CrossRef]
- Zhu, J.; Song, N.; Sun, S.; Yang, W.; Zhao, H.; Song, W.; Lai, J. Efficiency and inheritance of targeted mutagenesis in maize using CRISPR-Cas9. J. Genet. Genomics 2016, 43, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Yadava, P.; Kumar, K.; Singh, I. Insights into maize genome editing via CRISPR/Cas9. Physiol. Mol. Biol. Plants 2018, 24, 175–183. [Google Scholar] [CrossRef]
- Nakayasu, M.; Akiyama, R.; Lee, H.J.; Osakabe, K.; Osakabe, Y.; Watanabe, B.; Sugimoto, Y.; Umemoto, N.; Saito, K.; Muranaka, T.; et al. Generation of α-solanine-free hairy roots of potato by CRISPR/Cas9 mediated genome editing of the St16DOX gene. Plant Physiol. Biochem. 2018, 131, 70–77. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Al-Sadi, A.M.; Pour-Aboughadareh, A.; Burritt, D.J.; Tran, L.S.P. Genome editing using CRISPR/Cas9–targeted mutagenesis: An opportunity for yield improvements of crop plants grown under environmental stresses. Plant Physiol. Biochem. 2018, 131, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.; Bhat, J.A.; Mir, Z.A.; Sakina, A.; Ali, S.; Singh, A.K.; Tyagi, A.; Salgotra, R.K.; Dar, A.A.; Bhat, R. CRISPR/Cas approach: A new way of looking at plant-abiotic interactions. J. Plant Physiol. 2018, 224–225, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chen, L.; Liu, X.; Sun, S.; Wu, C.; Jiang, B.; Han, T.; Hou, W. CRISPR/Cas9-mediated genome editing in soybean hairy roots. PLoS ONE 2015, 10, e0136064. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, L.; Liu, X.; Guo, C.; Sun, S.; Wu, C.; Jiang, B.; Han, T.; Hou, W. CRISPR/Cas9-mediated targeted mutagenesis of GmFT2a delays flowering time in soybean. Plant Biotechnol. J. 2018, 16, 176–185. [Google Scholar] [CrossRef]




| Legume Crops | Growth Stage | Yield Loss | Reference |
|---|---|---|---|
| Soybean | Pod set | 73–82% | [67] |
| Reproductive phase | 46–71% | [70] | |
| Pod set | 45–50% | [69] | |
| Grain filling stage | 42% | [63] | |
| Chickpea | Reproductive phase | 45–69% | [71] |
| Ripening stage | 49–54% | [32] | |
| Anthesis stage | 27–40% | [48] | |
| Ripening stage | 50% | [72] | |
| Cowpea | Reproductive phase | 60% | [73] |
| Reproductive phase | 34–66% | [74] | |
| Pod filling stage | 29% | [75] | |
| Common bean | Reproductive phase | 58–87% | [76] |
| Pod filling stage | 40% | [77] | |
| Flowering stage | 49% | [78] | |
| Pigeonpea | Reproductive phase | 40–55% | [79] |
| Mung bean | Reproductive phase | 26% | [80] |
| Flowering stage | 31–57% | [81] | |
| Faba bean | Grain filling stage | 68% | [82] |
| Lentil | Pod development | 70% | [83] |
| Reproductive phase | 24% | [84] |
| Legume Crops | Trait | Reference |
|---|---|---|
| Soybean | Water use efficiency, root architecture | [135] |
| 100-grain weight | [67] | |
| Lateral root thickness | [136] | |
| Presence of dense leaf pubescence | [137] | |
| Carbohydrate storage and remobilization | [138] | |
| Chickpea | Prolific root system, Rooting depth, root length | [128,139] |
| Shoot biomass, leaf area index, canopy temperature decrease | [140] | |
| Smaller leaf area | [141] | |
| Grain size, early maturity and short stature | [142] | |
| Cowpea | Short duration and erect plant type | [86] |
| Common bean | Leaf RWC | [143] |
| Deeper and vigorous roots | [144] | |
| Canopy biomass, pod partitioning index, stem biomass reduction and pod harvest index | [145] | |
| Pigeonpea | Root and shoot biomass | [146] |
| Leaf area maintenance | [147] | |
| Mungbean | Dry matter partitioning | [148] |
| Faba bean | vigorous growth | [149] |
| Root growth | [150] | |
| Lentil | Dry root weight and root length | [133] |
| Legume Crops | Arbuscular Mycorrhizal Fungi/Bacterial Strains | Function | Reference |
|---|---|---|---|
| Soybean | Bradyrhizobium japonicum | Improve growth and yield | [201] |
| Bradyrhizobium japonicum | Improved grain yield | [202] | |
| Glomus mosseae, Glomus etunicatum | Maintenance of high leaf water potential | [203] | |
| Bradyrhizobium japonicum | Improved N contents | [203] | |
| Glomus intraradiecs | Protected against oxidative stress and root osmotic adjustment | [195] | |
| Pseudomonas cepacia | Early growth and ACC-diaminase production | [204] | |
| Bacillus spp. | Enhanced nodulation and pod formation | [205] | |
| Cow pea | Azospirillum spp. | Improve proton efflux activities | [191] |
| Glomus intraradiecs | Improve Stomatal conductance | [206] | |
| Common bean | Paenibacillus polymyxa and rhizobium tropici | Improved nodulation, N contents and plant growth | [200] |
| Glomus intraradiecs | Maintain root hydraulic conductance | [207] | |
| Gigaspora margarita | Dehydration maintenance | [208] | |
| Glomus intraradiecs | Improve Stomatal conductance | [208] | |
| Azospirillium brasilense | Improve root growth | [209] | |
| Bacillus spp. | Improved nodulation and root hair proliferation | [210,211] | |
| Green gram | Glomus mosseae, Glomus intraradiecs | Improved water-use efficiency | [212] |
| Pseudomonas putida | Improved root growth and ACC-diaminase production | [213] | |
| Pea | Varovorax paradoxus | Plant growth improvement through hormonal signaling | [187] |
| Pseudomonas spp. | Alleviating drought stress | [214,215] | |
| Lentil | Pseudomonas putida | Enhanced nodulation and plant growth | [214] |
| Legume Crops | Gene Transferred | Function | Reference |
|---|---|---|---|
| Soybean | PgTIP1 | Confers drought tolerance | [248] |
| GmDREB2 | Enhance drought tolerance | [249] | |
| GmRACK1 | Improve drought tolerance during vegetative growth | [250] | |
| AtABF3 | Improve drought tolerance | [251] | |
| GmFDL19 | Enhance drought tolerance | [252] | |
| GmSK1 | Enhance tolerance to drought | [253] | |
| GmNAC, GmDREB, GmZIP, ERF089 | Transcription factors | [238] | |
| DREB1A, rd29A | Transcription factors | [254] | |
| GmBIN2 | Enhance tolerance to drought | [255] | |
| GmCaM4 | Upreglate several drought-responsive genes | [256] | |
| CDPK | Enhance water permeability across the membrane | [257] | |
| GmHK, GmCLV1A, GmCLV1B, GmRLK1, GmRLK2, GmRLK3, GmRLK4 | Osmosensor | [258] | |
| Chickpea | Aquaporins | drought stress tolerance | [259] |
| DREB2A | Transcription factors | [260] | |
| MYB, WRKY, bZIP | Transcription factors | [261] | |
| MyB, AP2/ERF, XPB1 | Transcription factors | [262] | |
| Cowpea | VuPLD1, VuNCED1, CPRD8, CPRD12, CPRD14, CPRD22 | ABA-biosynthesis | [263] |
| Mungbean | VrbZIP | Drought-responsive gene | [264] |
| codA | Improve abiotic stress tolerance | [265] | |
| VrWRKY | Enhance abiotic stress tolerance | [266] | |
| Common bean | Asr1, Asr2 | ABA signaling pathway | [267] |
| PvLEA3 | Protein stabilization | [268] | |
| DREB2B | Non-ABA dependent response | [267] | |
| Pigeonpea | C.cajan_29830, C.cajan_33874 | Improve drought tolerance | [269] |
| WRKY, MyB, NF-Y | Transcription factors | [270] | |
| Broad bean | VfPIP1 | Aquaporin/water transport | [271] |
| Alfalfa | AtEDT1 | Confers drought tolerance | [272] |
| SPL13 | Improve drought tolerance | [273] | |
| CsLEA | Enhance tolerance to drought | [274] | |
| GsZFP1 | Confers drought tolerance | [275] | |
| codA | Enhance tolerance to drought | [276] | |
| HaHB11 | Confers tolerance to water deficit | [277] | |
| AVP1 | Enhance drought tolerance | [278] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research Progress and Perspective on Drought Stress in Legumes: A Review. Int. J. Mol. Sci. 2019, 20, 2541. https://doi.org/10.3390/ijms20102541
Nadeem M, Li J, Yahya M, Sher A, Ma C, Wang X, Qiu L. Research Progress and Perspective on Drought Stress in Legumes: A Review. International Journal of Molecular Sciences. 2019; 20(10):2541. https://doi.org/10.3390/ijms20102541
Chicago/Turabian StyleNadeem, Muhammad, Jiajia Li, Muhammad Yahya, Alam Sher, Chuanxi Ma, Xiaobo Wang, and Lijuan Qiu. 2019. "Research Progress and Perspective on Drought Stress in Legumes: A Review" International Journal of Molecular Sciences 20, no. 10: 2541. https://doi.org/10.3390/ijms20102541
APA StyleNadeem, M., Li, J., Yahya, M., Sher, A., Ma, C., Wang, X., & Qiu, L. (2019). Research Progress and Perspective on Drought Stress in Legumes: A Review. International Journal of Molecular Sciences, 20(10), 2541. https://doi.org/10.3390/ijms20102541