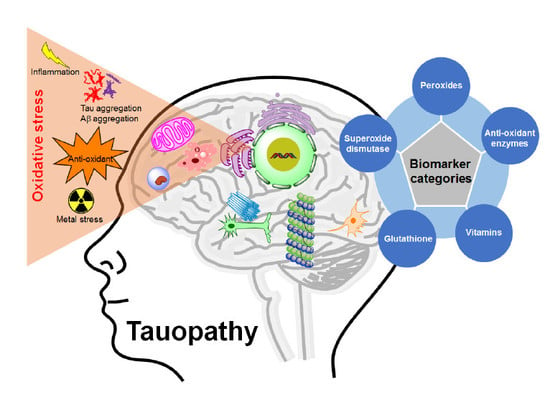
Crosstalk between Oxidative Stress and Tauopathy
Abstract
:1. Tau Protein and its Pathogenicity
2. Causes of Tauopathies
3. Oxidative Stress and Its Relation to Tauopathies
4. Oxidative Stress and Reactive Oxygen Species
5. Major Sources of Oxidative Stress (OS)
6. Reactive Oxygen Species (ROS) Production in the Body
6.1. Mitochondria
6.2. Endoplasmic Reticulum
6.3. Peroxisomes
6.4. Microglia
7. Chemical Properties of ROS
7.1. Electronic Configuration of ROS
7.2. Reactivity Trends of ROS
8. ROS Generating Agents in Tauopathies
8.1. Tau Aggregation
8.2. Amyloid-Beta Aggregation
8.3. Metals
8.4. Inflammation
8.5. Anti-Oxidant
9. Evaluation of ROS in Tauopathies
10. Fluorescence Probes for ROS Detection
11. Controlling of ROS as Therapeutic Approaches of Tauopathies
11.1. Antioxidant Pathway
11.2. Catalase
11.3. Vitamin
11.4. Metal Chelator
12. Conclusions
Funding
Conflicts of Interest
Abbreviations
| •OH | Hydroxyl radical |
| 1O2 | Singlet oxygen |
| 8-OHdG | 8-Hydroxy-2’-deoxyguanosine |
| 8-OHG | 8-Hydroxy-2’-guanosine |
| Aβ | Amyloid beta’ Abeta |
| AD | Alzheimer’s disease |
| Al | Aluminium |
| ATP | Adenosine triphosphate |
| CBD | Corticobasal degeneration |
| CNS | Central nervous system |
| CO3•− | Carbonate radical |
| CoQ10 | Coenzyme Q10 |
| COX | Cytochrome oxidase |
| Cu | Copper |
| DMSO | Dimethyl sulfoxide |
| ER | Endoplasmic reticulum |
| ETC | Electron transport chain |
| FDA | US Food and Drug Administration |
| Fe | Iron |
| FTD | Frontotemporal dementia |
| FTDP-17 | FTD with parkinsonism linked to chromosome-17 |
| FTLD | Frontotemporal lobar degeneration |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| GST | Glutathione S transferase |
| H2O2 | Hydrogen peroxide |
| Hcy | Homocysteine |
| 4-HNE | 4-Hydroxynonenal |
| HO-1 | Heme oxygenase-1 |
| HO2 | Hydroperoxyl radical |
| HOBr | Hypobromous |
| HOCl | Hypochlorous |
| HOI | Hypoiodous acids |
| Hr | Hour |
| KGDHC | α-Ketogluterate dehydrogenase complex |
| MAPK | Mitogen-activated protein kinase |
| MB | Methylene blue; methylthioninium chloride |
| NFT | Neurofibrillary tangle |
| NO or NO• | Nitric oxide radical |
| NO2− | Nitrite |
| NO2• | Nitrogen dioxide radical |
| O2− or O2•− | Superoxide anion radical |
| O3 | Ozone |
| ONOO− | Peroxynitrite |
| OS | Oxidative stress |
| PD | Parkinson’s disease |
| PDHC | Pyruvate dehydrogenase complex |
| PiD | Pick’s disease |
| PSP | Progressive supranuclear palsy |
| PTM | Posttranslational modification |
| RNS | Reactive nitrogen species |
| RO• | Alkoxyl radical |
| ROI | Reactive oxygen intermediate |
| ROO• | Peroxyl radical |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SQ•− | Semiquinone radical |
| Zn | Zinc |
References
- Shin, R.W.; Iwaki, T.; Kitamoto, T.; Tateishi, J. Hydrated autoclave pretreatment enhances tau immunoreactivity in formalin-fixed normal and Alzheimer’s disease brain tissues. Lab. Investig. 1991, 64, 693–702. [Google Scholar]
- Cleveland, D.W.; Hwo, S.Y.; Kirschner, M.W. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J. Mol. Biol. 1977, 116, 227–247. [Google Scholar] [CrossRef]
- Kolarova, M.; Garcia-Sierra, F.; Bartos, A.; Ricny, J.; Ripova, D. Structure and pathology of tau protein in Alzheimer disease. Int. J. Alzheimers Dis. 2012, 2012, 731526. [Google Scholar] [CrossRef]
- Mazanetz, M.P.; Fischer, P.M. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat. Rev. Drug Discov. 2007, 6, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Hoshi, M.; Nishida, E.; Minami, Y.; Sakai, H. Binding of microtubule-associated protein 2 and tau to the intermediate filament reassembled from neurofilament 70-kDa subunit protein. Its regulation by calmodulin. J. Biol. Chem. 1986, 261, 13026–13030. [Google Scholar]
- Jung, D.; Filliol, D.; Miehe, M.; Rendon, A. Interaction of brain mitochondria with microtubules reconstituted from brain tubulin and MAP2 or TAU. Cell Motil. Cytoskeleton 1993, 24, 245–255. [Google Scholar] [CrossRef]
- Mandelkow, E.M.; Stamer, K.; Vogel, R.; Thies, E.; Mandelkow, E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol. Aging 2003, 24, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Obulesu, M.; Venu, R.; Somashekhar, R. Tau mediated neurodegeneration: An insight into Alzheimer’s disease pathology. Neurochem. Res. 2011, 36, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Buee, L.; Bussiere, T.; Buee-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 2000, 33, 95–130. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Goedert, M. Tau pathology and neurodegeneration. Lancet Neurol. 2013, 12, 609–622. [Google Scholar] [CrossRef]
- Alavi Naini, S.M.; Soussi-Yanicostas, N. Tau Hyperphosphorylation and Oxidative Stress, a Critical Vicious Circle in Neurodegenerative Tauopathies? Oxid. Med. Cell Longev. 2015, 2015, 151979. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, F.; Wang, D.; Li, C.; Fu, Y.; He, W.; Zhang, J. Tau Pathology in Parkinson’s Disease. Front. Neurol. 2018, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Kim, D.; Yu, Y.H.; Lim, S.; Kim, D.J.; Chang, Y.T.; Ha, H.H.; Kim, Y.K. Inhibition of tau aggregation by a rosamine derivative that blocks tau intermolecular disulfide cross-linking. Amyloid 2014, 21, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Haque, M.M.; Kim, D.; Kim, D.J.; Kim, Y.K. Cell-based Models To Investigate Tau Aggregation. Comput. Struct. Biotechnol. J. 2014, 12, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrell, R.W.; Gooptu, B. Conformational changes and disease—Serpins, prions and Alzheimer’s. Curr. Opin. Struct. Biol. 1998, 8, 799–809. [Google Scholar] [CrossRef]
- Binder, L.I.; Guillozet-Bongaarts, A.L.; Garcia-Sierra, F.; Berry, R.W. Tau, tangles, and Alzheimer’s disease. Biochim. Biophys. Acta 2005, 1739, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Schweers, O.; Mandelkow, E.M.; Biernat, J.; Mandelkow, E. Oxidation of cysteine-322 in the repeat domain of microtubule-associated protein tau controls the in vitro assembly of paired helical filaments. Proc. Natl. Acad. Sci. USA 1995, 92, 8463–8467. [Google Scholar] [CrossRef] [PubMed]
- Wischik, C.M.; Novak, M.; Edwards, P.C.; Klug, A.; Tichelaar, W.; Crowther, R.A. Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc. Natl. Acad. Sci. USA 1988, 85, 4884–4888. [Google Scholar] [CrossRef]
- Groeger, G.; Quiney, C.; Cotter, T.G. Hydrogen peroxide as a cell-survival signaling molecule. Antioxid. Redox Signal. 2009, 11, 2655–2671. [Google Scholar] [CrossRef]
- Patten, D.A.; Germain, M.; Kelly, M.A.; Slack, R.S. Reactive oxygen species: Stuck in the middle of neurodegeneration. J. Alzheimers Dis. 2010, 20 (Suppl. 2), S357–S367. [Google Scholar] [CrossRef]
- Dasuri, K.; Zhang, L.; Keller, J.N. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radic. Biol. Med. 2013, 62, 170–185. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Ferreira, M.E.; de Vasconcelos, A.S.; da Costa Vilhena, T.; da Silva, T.L.; da Silva Barbosa, A.; Gomes, A.R.; Dolabela, M.F.; Percario, S. Oxidative Stress in Alzheimer’s Disease: Should We Keep Trying Antioxidant Therapies? Cell Mol. Neurobiol. 2015, 35, 595–614. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lee, H.G.; Casadesus, G.; Avila, J.; Drew, K.; Perry, G.; Smith, M.A. Oxidative imbalance in Alzheimer’s disease. Mol. Neurobiol. 2005, 31, 205–217. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Sun, Q.; Chen, S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef]
- Resende, R.; Moreira, P.I.; Proenca, T.; Deshpande, A.; Busciglio, J.; Pereira, C.; Oliveira, C.R. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic. Biol. Med. 2008, 44, 2051–2057. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef]
- Stamer, K.; Vogel, R.; Thies, E.; Mandelkow, E.; Mandelkow, E.M. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J. Cell Biol. 2002, 156, 1051–1063. [Google Scholar] [CrossRef] [Green Version]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.M.; Iwata, N.; Saido, T.C.; Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef]
- David, D.C.; Hauptmann, S.; Scherping, I.; Schuessel, K.; Keil, U.; Rizzu, P.; Ravid, R.; Drose, S.; Brandt, U.; Muller, W.E.; et al. Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. J. Biol. Chem. 2005, 280, 23802–23814. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid beta-peptide (1-42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef]
- Ashabi, G.; Ahmadiani, A.; Abdi, A.; Abraki, S.B.; Khodagholi, F. Time course study of Abeta formation and neurite outgrowth disruption in differentiated human neuroblastoma cells exposed to H2O2: Protective role of autophagy. Toxicol. In Vitro 2013, 27, 1780–1788. [Google Scholar] [CrossRef]
- Hauser, D.N.; Hastings, T.G. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease and monogenic parkinsonism. Neurobiol. Dis. 2013, 51, 35–42. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. SnapShot: Reactive oxygen intermediates (ROI). Cell 2010, 140, 951–951.e2. [Google Scholar] [CrossRef] [PubMed]
- Krumova, K.; Cosa, G. Overview of reactive oxygen species. Singlet Oxygen: Appl. Biosci. Nanosci. 2016, 1, 1–21. [Google Scholar]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504. [Google Scholar] [CrossRef]
- Chong, W.; Shastri, M.; Eri, R. Endoplasmic reticulum stress and oxidative stress: A vicious nexus implicated in bowel disease pathophysiology. Int. J. Mol. Sci. 2017, 18, 771. [Google Scholar] [CrossRef] [PubMed]
- Walling, C. Fenton’s reagent revisited. Acc. Chem. Res. 1975, 8, 125–131. [Google Scholar] [CrossRef]
- Imlay, J.A. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef] [PubMed]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813. [Google Scholar] [CrossRef]
- Leach, J.K.; Van Tuyle, G.; Lin, P.-S.; Schmidt-Ullrich, R.; Mikkelsen, R.B. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001, 61, 3894–3901. [Google Scholar] [PubMed]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Raina, A.K.; Lee, H.G.; Casadesus, G.; Smith, M.A.; Perry, G. Oxidative stress signalling in Alzheimer’s disease. Brain Res 2004, 1000, 32–39. [Google Scholar] [CrossRef]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014, 42 (Suppl. 3), S125–S152. [Google Scholar] [CrossRef]
- Bhandary, B.; Marahatta, A.; Kim, H.R.; Chae, H.J. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int. J. Mol. Sci. 2012, 14, 434–456. [Google Scholar] [CrossRef]
- Buettner, G.R. The pecking order of free radicals and antioxidants: Lipid peroxidation, α-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef]
- Wood, P.M. The potential diagram for oxygen at pH 7. Biochem. J. 1988, 253, 287–289. [Google Scholar] [CrossRef] [Green Version]
- Gamblin, T.C.; King, M.E.; Kuret, J.; Berry, R.W.; Binder, L.I. Oxidative regulation of fatty acid-induced tau polymerization. Biochemistry 2000, 39, 14203–14210. [Google Scholar] [CrossRef]
- Cente, M.; Filipcik, P.; Pevalova, M.; Novak, M. Expression of a truncated tau protein induces oxidative stress in a rodent model of tauopathy. Eur. J. Neurosci. 2006, 24, 1085–1090. [Google Scholar] [CrossRef]
- Chen, L.; Na, R.; Gu, M.; Richardson, A.; Ran, Q. Lipid peroxidation up-regulates BACE1 expression in vivo: A possible early event of amyloidogenesis in Alzheimer’s disease. J. Neurochem. 2008, 107, 197–207. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Picciano, M.; La Francois, J.; Duff, K. Fibrillar beta-amyloid evokes oxidative damage in a transgenic mouse model of Alzheimer’s disease. Neuroscience 2001, 104, 609–613. [Google Scholar] [CrossRef]
- Murakami, K.; Murata, N.; Noda, Y.; Tahara, S.; Kaneko, T.; Kinoshita, N.; Hatsuta, H.; Murayama, S.; Barnham, K.J.; Irie, K.; et al. SOD1 (copper/zinc superoxide dismutase) deficiency drives amyloid beta protein oligomerization and memory loss in mouse model of Alzheimer disease. J. Biol. Chem. 2011, 286, 44557–44568. [Google Scholar] [CrossRef]
- Deibel, M.A.; Ehmann, W.D.; Markesbery, W.R. Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: Possible relation to oxidative stress. J. Neurol. Sci. 1996, 143, 137–142. [Google Scholar] [CrossRef]
- Crapper, D.R.; Krishnan, S.S.; Dalton, A.J. Brain aluminum distribution in Alzheimer’s disease and experimental neurofibrillary degeneration. Science 1973, 180, 511–513. [Google Scholar] [CrossRef]
- Mandrekar-Colucci, S.; Landreth, G.E. Microglia and inflammation in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2010, 9, 156–167. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Rees, H.D.; Weintraub, S.T.; Levey, A.I.; Chin, L.S.; Li, L. Oxidative modifications and aggregation of Cu,Zn-superoxide dismutase associated with Alzheimer and Parkinson diseases. J. Biol. Chem. 2005, 280, 11648–11655. [Google Scholar] [CrossRef]
- Martinez, A.; Carmona, M.; Portero-Otin, M.; Naudi, A.; Pamplona, R.; Ferrer, I. Type-dependent oxidative damage in frontotemporal lobar degeneration: Cortical astrocytes are targets of oxidative damage. J. Neuropathol. Exp. Neurol. 2008, 67, 1122–1136. [Google Scholar] [CrossRef] [PubMed]
- De Leo, M.E.; Borrello, S.; Passantino, M.; Palazzotti, B.; Mordente, A.; Daniele, A.; Filippini, V.; Galeotti, T.; Masullo, C. Oxidative stress and overexpression of manganese superoxide dismutase in patients with Alzheimer’s disease. Neurosci. Lett. 1998, 250, 173–176. [Google Scholar] [CrossRef]
- Dias-Santagata, D.; Fulga, T.A.; Duttaroy, A.; Feany, M.B. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J. Clin. Investig. 2007, 117, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.; Olanow, C.W. Understanding cell death in Parkinson’s disease. Ann. Neurol. 1998, 44, S72–S84. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Squitti, R.; Pasqualetti, P.; Marchese, E.; Cassetta, E.; Forastiere, E.; Rotilio, G.; Rossini, P.M.; Finazzi-Agro, A. Red blood cell copper, zinc superoxide dismutase activity is higher in Alzheimer’s disease and is decreased by D-penicillamine. Neurosci. Lett 2002, 329, 137–140. [Google Scholar] [CrossRef]
- Perry, T.L.; Hansen, S.; Jones, K. Brain amino acids and glutathione in progressive supranuclear palsy. Neurology 1988, 38, 943–946. [Google Scholar] [CrossRef]
- Fitzmaurice, P.S.; Ang, L.; Guttman, M.; Rajput, A.H.; Furukawa, Y.; Kish, S.J. Nigral glutathione deficiency is not specific for idiopathic Parkinson’s disease. Mov. Disord. 2003, 18, 969–976. [Google Scholar] [CrossRef]
- Wojsiat, J.; Zoltowska, K.M.; Laskowska-Kaszub, K.; Wojda, U. Oxidant/Antioxidant Imbalance in Alzheimer’s Disease: Therapeutic and Diagnostic Prospects. Oxid. Med. Cell Longev. 2018, 2018, 6435861. [Google Scholar] [CrossRef]
- Youssef, P.; Chami, B.; Lim, J.; Middleton, T.; Sutherland, G.T.; Witting, P.K. Evidence supporting oxidative stress in a moderately affected area of the brain in Alzheimer’s disease. Sci. Rep. 2018, 8, 11553. [Google Scholar] [CrossRef] [PubMed]
- Nikam, S.; Nikam, P.; Ahaley, S.K.; Sontakke, A.V. Oxidative stress in Parkinson’s disease. Indian J. Clin. Biochem. 2009, 24, 98–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, L.L.; Quaglio, N.B.; de Souza, G.T.; Garcia, R.T.; Dati, L.M.; Moreira, W.L.; Loureiro, A.P.; de Souza-Talarico, J.N.; Smid, J.; Porto, C.S.; et al. Peripheral oxidative stress biomarkers in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2011, 26, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K.; Matsubara, K.; Kobayashi, S. Aging and oxidative stress in progressive supranuclear palsy. Eur. J. Neurol. 2006, 13, 89–92. [Google Scholar] [CrossRef]
- Damier, P.; Hirsch, E.C.; Zhang, P.; Agid, Y.; Javoy-Agid, F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience 1993, 52, 1–6. [Google Scholar] [CrossRef]
- Odetti, P.; Garibaldi, S.; Norese, R.; Angelini, G.; Marinelli, L.; Valentini, S.; Menini, S.; Traverso, N.; Zaccheo, D.; Siedlak, S.; et al. Lipoperoxidation is selectively involved in progressive supranuclear palsy. J. Neuropathol. Exp. Neurol. 2000, 59, 393–397. [Google Scholar] [CrossRef]
- Gerst, J.L.; Siedlak, S.L.; Nunomura, A.; Castellani, R.; Perry, G.; Smith, M.A. Role of oxidative stress in frontotemporal dementia. Dement. Geriatr. Cogn. Disord. 1999, 10 (Suppl. 1), 85–87. [Google Scholar] [CrossRef]
- Sudha, K.; Rao, A.V.; Rao, S.; Rao, A. Free radical toxicity and antioxidants in Parkinson’s disease. Neurol. India 2003, 51, 60–62. [Google Scholar] [PubMed]
- Albers, D.S.; Augood, S.J.; Martin, D.M.; Standaert, D.G.; Vonsattel, J.P.; Beal, M.F. Evidence for oxidative stress in the subthalamic nucleus in progressive supranuclear palsy. J. Neurochem. 1999, 73, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Zarkovic, K. 4-Hydroxynonenal and neurodegenerative diseases. Mol. Asp. Med. 2003, 24, 293–303. [Google Scholar] [CrossRef]
- Yoritaka, A.; Hattori, N.; Uchida, K.; Tanaka, M.; Stadtman, E.R.; Mizuno, Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc. Natl. Acad. Sci. USA 1996, 93, 2696–2701. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Yamada, M. Vitamin A and Alzheimer’s disease. Geriatr. Gerontol. Int. 2012, 12, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.Y.; Lee, K.M.; Kim, Y.; Cha, M.Y.; Kang, S.; Baik, S.H.; Lee, H.; Park, R.; Mook-Jung, I. High-dose of vitamin C supplementation reduces amyloid plaque burden and ameliorates pathological changes in the brain of 5XFAD mice. Cell Death Dis. 2014, 5, e1083. [Google Scholar] [CrossRef] [PubMed]
- Lloret, A.; Esteve, D.; Monllor, P.; Cervera-Ferri, A.; Lloret, A. The Effectiveness of Vitamin E Treatment in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 879. [Google Scholar] [CrossRef]
- Castellani, R.; Smith, M.A.; Richey, P.L.; Kalaria, R.; Gambetti, P.; Perry, G. Evidence for oxidative stress in Pick disease and corticobasal degeneration. Brain Res 1995, 696, 268–271. [Google Scholar] [CrossRef]
- Ihara, Y.; Chuda, M.; Kuroda, S.; Hayabara, T. Hydroxyl radical and superoxide dismutase in blood of patients with Parkinson’s disease: Relationship to clinical data. J. Neurol. Sci. 1999, 170, 90–95. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 2011, 6, e28032. [Google Scholar] [CrossRef]
- Kikuchi, A.; Takeda, A.; Onodera, H.; Kimpara, T.; Hisanaga, K.; Sato, N.; Nunomura, A.; Castellani, R.J.; Perry, G.; Smith, M.A.; et al. Systemic increase of oxidative nucleic acid damage in Parkinson’s disease and multiple system atrophy. Neurobiol. Dis. 2002, 9, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Roostaei, T.; Felsky, D.; Nazeri, A.; de Jager, P.L.; Schneider, J.A.; Bennett, D.A.; Voineskos, A.N.; Alzheimer’s Disease Neuroimaging Initiative. Genetic influence of plasma homocysteine on Alzheimer’s disease. Neurobiol. Aging 2018, 62, 243.e7–243.e14. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Guan, P.P.; Wang, T.; Yu, X.; Guo, J.J.; Wang, Z.Y. Aggravation of Alzheimer’s disease due to the COX-2-mediated reciprocal regulation of IL-1beta and Abeta between glial and neuron cells. Aging Cell 2014, 13, 605–615. [Google Scholar] [CrossRef]
- Martin, E.; Rosenthal, R.E.; Fiskum, G. Pyruvate dehydrogenase complex: Metabolic link to ischemic brain injury and target of oxidative stress. J. Neurosci. Res. 2005, 79, 240–247. [Google Scholar] [CrossRef]
- Shi, Q.; Xu, H.; Kleinman, W.A.; Gibson, G.E. Novel functions of the alpha-ketoglutarate dehydrogenase complex may mediate diverse oxidant-induced changes in mitochondrial enzymes associated with Alzheimer’s disease. Biochim. Biophys. Acta 2008, 1782, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, M.; Yuan, Z. Methods for the detection of reactive oxygen species. Anal. Methods 2018, 10, 4625–4638. [Google Scholar] [CrossRef]
- Chen, X.; Wang, F.; Hyun, J.Y.; Wei, T.; Qiang, J.; Ren, X.; Shin, I.; Yoon, J. Recent progress in the development of fluorescent, luminescent and colorimetric probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2016, 45, 2976–3016. [Google Scholar] [CrossRef]
- Andina, D.; Leroux, J.C.; Luciani, P. Ratiometric fluorescent probes for the detection of reactive oxygen species. Chemistry 2017, 23, 13549–13573. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, E.; Lima, J.L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Lo, L.-C.; Chu, C.-Y. Development of highly selective and sensitive probes for hydrogen peroxide. Chem. Commun. 2003, 7, 2728–2729. [Google Scholar] [CrossRef]
- Chang, M.C.; Pralle, A.; Isacoff, E.Y.; Chang, C.J. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J. Am. Chem. Soc. 2004, 126, 15392–15393. [Google Scholar] [CrossRef] [PubMed]
- Lippert, A.R.; van de Bittner, G.C.; Chang, C.J. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc. Chem. Res. 2011, 44, 793–804. [Google Scholar] [CrossRef]
- Kuivila, H.G.; Armour, A.G. Electrophilic displacement reactions. IX. Effects of substituents on rates of reactions between hydrogen peroxide and benzeneboronic acid1-3. J. Am. Chem. Soc. 1957, 79, 5659–5662. [Google Scholar] [CrossRef]
- Medvedeva, N.; Martin, V.V.; Weis, A.L.; Likhtenshten, G.I. Dual fluorophore-nitronyl probe for investigation of superoxide dynamics and antioxidant status of biological systems. J. Photochem. Photobiol. A Chem. 2004, 163, 45–51. [Google Scholar] [CrossRef]
- Afanasev, I. On mechanism of superoxide signaling under physiological and pathophysiological conditions. Med. Hypotheses 2005, 64, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, X.; Tang, B. A phosphinate-based red fluorescent probe for imaging the superoxide radical anion generated by RAW264. 7 macrophages. ChemBioChem 2007, 8, 453–458. [Google Scholar] [CrossRef]
- Hu, J.J.; Wong, N.-K.; Ye, S.; Chen, X.; Lu, M.-Y.; Zhao, A.Q.; Guo, Y.; Ma, A.C.-H.; Leung, A.Y.-H.; Shen, J. Fluorescent probe HKSOX-1 for imaging and detection of endogenous superoxide in live cells and in vivo. J. Am. Chem. Soc. 2015, 137, 6837–6843. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Yamamoto, K.; Nomura, Y.; Kohno, I.; Hafsi, L.; Ueda, N.; Yoshida, S.; Fukuda, M.; Fukuyasu, Y.; Yamauchi, Y. A design of fluorescent probes for superoxide based on a nonredox mechanism. J. Am. Chem. Soc. 2005, 127, 68–69. [Google Scholar] [CrossRef]
- Murale, D.P.; Kim, H.; Choi, W.S.; Churchill, D.G. Highly selective excited state intramolecular proton transfer (ESIPT)-based superoxide probing. Organ. Lett. 2013, 15, 3946–3949. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.C.; Murale, D.P.; Jang, S.Y.; Haque, M.M.; Seo, M.; Lee, S.; Woo, D.H.; Kwon, J.; Song, C.S.; Kim, Y.K. Discrimination of Avian Influenza Virus Subtypes using Host-Cell Infection Fingerprinting by a Sulfinate-based Fluorescence Superoxide Probe. Angew. Chem. Int. Ed. 2018, 57, 9716–9721. [Google Scholar] [CrossRef]
- You, Y.; Nam, W. Designing photoluminescent molecular probes for singlet oxygen, hydroxyl radical, and iron–oxygen species. Chem. Sci. 2014, 5, 4123–4135. [Google Scholar] [CrossRef]
- Ohashi, T.; Mizutani, A.; Murakami, A.; Kojo, S.; Ishii, T.; Taketani, S. Rapid oxidation of dichlorodihydrofluorescin with heme and hemoproteins: Formation of the fluorescein is independent of the generation of reactive oxygen species. FEBS Lett. 2002, 511, 21–27. [Google Scholar] [CrossRef]
- Kundu, K.; Knight, S.F.; Willett, N.; Lee, S.; Taylor, W.R.; Murthy, N. Hydrocyanines: A class of fluorescent sensors that can image reactive oxygen species in cell culture, tissue, and in vivo. Angew. Chem. Int. Ed. 2009, 48, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Song, J. Ratiometric fluorescent detection of intracellular hydroxyl radicals based on a hybrid coumarin–cyanine platform. Chem. Commun. 2010, 46, 7930–7932. [Google Scholar] [CrossRef]
- Aliaga, C.; Fuentealba, P.; Rezende, M.C.; Cárdenas, C. Mechanism of fluorophore quenching in a pre-fluorescent nitroxide probe: A theoretical illustration. Chem. Phys. Lett. 2014, 593, 89–92. [Google Scholar] [CrossRef]
- Kang, P.; Foote, C.S. Formation of transient intermediates in low-temperature photosensitized oxidation of an 8-13C-guanosine derivative. J. Am. Chem. Soc. 2002, 124, 4865–4873. [Google Scholar] [CrossRef]
- Umezawa, N.; Tanaka, K.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Nagano, T. Novel fluorescent probes for singlet oxygen. Angew. Chem. Int. Ed. 1999, 38, 2899–2901. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Jangili, P.; Sharma, A.; Li, W.; Hou, J.-T.; Qin, C.; Yoon, J.; Kim, J.S. Design and applications of fluorescent detectors for peroxynitrite. Coord. Chem. Rev. 2018, 374, 36–54. [Google Scholar] [CrossRef]
- Wang, B.; Yu, F.; Li, P.; Sun, X.; Han, K. A BODIPY fluorescence probe modulated by selenoxide spirocyclization reaction for peroxynitrite detection and imaging in living cells. Dyes Pigment. 2013, 96, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Manjare, S.T.; Kim, Y.; Churchill, D.G. Selenium-and tellurium-containing fluorescent molecular probes for the detection of biologically important analytes. Acc. Chem. Res. 2014, 47, 2985–2998. [Google Scholar] [CrossRef]
- Lou, Z.; Li, P.; Han, K. Redox-responsive fluorescent probes with different design strategies. Acc. Chem. Res. 2015, 48, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Iverson, N.; Hofferber, E.; Stapleton, J. Nitric Oxide Sensors for Biological Applications. Chemosensors 2018, 6, 8. [Google Scholar] [CrossRef]
- Vegesna, G.K.; Sripathi, S.R.; Zhang, J.; Zhu, S.; He, W.; Luo, F.-T.; Jahng, W.J.; Frost, M.; Liu, H. Highly water-soluble BODIPY-based fluorescent probe for sensitive and selective detection of nitric oxide in living cells. ACS Appl. Mater. Interfaces 2013, 5, 4107–4112. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y. A water-soluble sulfonate-BODIPY based fluorescent probe for selective detection of HOCl/OCl− in aqueous media. Analyst 2014, 139, 2986–2989. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Yuan, P.; Yang, J.; Xu, Y.; Grutzendler, J.; Shao, Y.; Moore, A.; Ran, C. Oxalate-curcumin-based probe for micro- and macroimaging of reactive oxygen species in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 12384–12389. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.; Liang, S.H.; Xu, Y.; Moore, A.; Ran, C. Imaging hydrogen peroxide in Alzheimer’s disease via cascade signal amplification. Sci. Rep. 2016, 6, 35613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Yang, J.; Li, Y.; Xu, Y.; Ran, C. Near-infrared Fluorescence Ocular Imaging (NIRFOI) of Alzheimer’s Disease. Mol. Imaging Biol. 2019, 21, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mu, X.Y.; Yang, J.; Liang, Y.Y.; Zhang, X.D.; Ming, D. Brain imaging with near-infrared fluorophores. Coord. Chem. Rev. 2019, 380, 550–571. [Google Scholar] [CrossRef]
- Needham, L.M.; Weber, J.; Fyfe, J.W.B.; Kabia, O.M.; Do, D.T.; Klimont, E.; Zhang, Y.; Rodrigues, M.; Dobson, C.M.; Ghandi, S.; et al. Bifunctional fluorescent probes for detection of amyloid aggregates and reactive oxygen species. R. Soc. Open Sci. 2018, 5, 171399. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Liu, X.; Tang, B.; Yang, G.; Yang, Y.; An, L. Design of a phosphinate-based fluorescent probe for superoxide detection in mouse peritoneal macrophages. Chemistry 2007, 13, 1411–1416. [Google Scholar] [CrossRef]
- Yang, X.F.; Guo, X.Q. Investigation of the anthracene-nitroxide hybrid molecule as a probe for hydroxyl radicals. Analyst 2001, 126, 1800–1804. [Google Scholar] [CrossRef]
- Leikert, J.F.; Rathel, T.R.; Muller, C.; Vollmar, A.M.; Dirsch, V.M. Reliable in vitro measurement of nitric oxide released from endothelial cells using low concentrations of the fluorescent probe 4,5-diaminofluorescein. FEBS Lett. 2001, 506, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Kim, H.S.; Cho, E.K.; Kwon, B.Y.; Phark, S.; Hwang, K.W.; Sul, D. Curcumin protected PC12 cells against beta-amyloid-induced toxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Food Chem. Toxicol. 2008, 46, 2881–2887. [Google Scholar] [CrossRef]
- Stack, C.; Jainuddin, S.; Elipenahli, C.; Gerges, M.; Starkova, N.; Starkov, A.A.; Jove, M.; Portero-Otin, M.; Launay, N.; Pujol, A.; et al. Methylene blue upregulates Nrf2/ARE genes and prevents tau-related neurotoxicity. Hum. Mol. Genet. 2014, 23, 3716–3732. [Google Scholar] [CrossRef] [Green Version]
- Elipenahli, C.; Stack, C.; Jainuddin, S.; Gerges, M.; Yang, L.; Starkov, A.; Beal, M.F.; Dumont, M. Behavioral improvement after chronic administration of coenzyme Q10 in P301S transgenic mice. J. Alzheimers Dis. 2012, 28, 173–182. [Google Scholar] [CrossRef]
- Morroni, F.; Tarozzi, A.; Sita, G.; Bolondi, C.; Zolezzi Moraga, J.M.; Cantelli-Forti, G.; Hrelia, P. Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson’s disease. Neurotoxicology 2013, 36, 63–71. [Google Scholar] [CrossRef]
- Takenouchi, T.; Sekiyama, K.; Sekigawa, A.; Fujita, M.; Waragai, M.; Sugama, S.; Iwamaru, Y.; Kitani, H.; Hashimoto, M. P2X7 receptor signaling pathway as a therapeutic target for neurodegenerative diseases. Arch. Immunol. Ther. Exp. (Warsz.) 2010, 58, 91–96. [Google Scholar] [CrossRef]
- Clausen, A.; Xu, X.; Bi, X.; Baudry, M. Effects of the superoxide dismutase/catalase mimetic EUK-207 in a mouse model of Alzheimer’s disease: Protection against and interruption of progression of amyloid and tau pathology and cognitive decline. J. Alzheimers Dis. 2012, 30, 183–208. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, M.; Li, C.; Jiang, X.; Su, Y.; Zhang, Y. Benefits of Vitamins in the Treatment of Parkinson’s Disease. Oxid. Med. Cell Longev. 2019, 2019, 9426867. [Google Scholar] [CrossRef]
- Cente, M.; Filipcik, P.; Mandakova, S.; Zilka, N.; Krajciova, G.; Novak, M. Expression of a truncated human tau protein induces aqueous-phase free radicals in a rat model of tauopathy: Implications for targeted antioxidative therapy. J. Alzheimers Dis. 2009, 17, 913–920. [Google Scholar] [CrossRef]
- Nakashima, H.; Ishihara, T.; Yokota, O.; Terada, S.; Trojanowski, J.Q.; Lee, V.M.; Kuroda, S. Effects of alpha-tocopherol on an animal model of tauopathies. Free Radic. Biol. Med. 2004, 37, 176–186. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Tamtaji, O.R.; Dadgostar, E.; Daneshvar Kakhaki, R.; Bahmani, F.; Abolhassani, J.; Aarabi, M.H.; Kouchaki, E.; Memarzadeh, M.R.; Asemi, Z. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Neurochem. Int. 2017, 108, 183–189. [Google Scholar] [CrossRef]
- Grossi, C.; Francese, S.; Casini, A.; Rosi, M.C.; Luccarini, I.; Fiorentini, A.; Gabbiani, C.; Messori, L.; Moneti, G.; Casamenti, F. Clioquinol decreases amyloid-beta burden and reduces working memory impairment in a transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2009, 17, 423–440. [Google Scholar] [CrossRef]
- Faux, N.G.; Ritchie, C.W.; Gunn, A.; Rembach, A.; Tsatsanis, A.; Bedo, J.; Harrison, J.; Lannfelt, L.; Blennow, K.; et al. PBT2 rapidly improves cognition in Alzheimer’s Disease: Additional phase II analyses. J. Alzheimers Dis. 2010, 20, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Crouch, P.J.; Hung, L.W.; Adlard, P.A.; Cortes, M.; Lal, V.; Filiz, G.; Perez, K.A.; Nurjono, M.; Caragounis, A.; Du, T.; et al. Increasing Cu bioavailability inhibits Abeta oligomers and tau phosphorylation. Proc. Natl. Acad. Sci. USA 2009, 106, 381–386. [Google Scholar] [CrossRef]
- Crapper McLachlan, D.R.; Dalton, A.J.; Kruck, T.P.; Bell, M.Y.; Smith, W.L.; Kalow, W.; Andrews, D.F. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 1991, 337, 1304–1308. [Google Scholar] [CrossRef]
- Lejri, I.; Grimm, A.; Miesch, M.; Geoffroy, P.; Eckert, A.; Mensah-Nyagan, A.G. Allopregnanolone and its analog BR 297 rescue neuronal cells from oxidative stress-induced death through bioenergetic improvement. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gonzalez, R.; Pascual, C.; Antequera, D.; Bolos, M.; Redondo, M.; Perez, D.I.; Perez-Grijalba, V.; Krzyzanowska, A.; Sarasa, M.; Gil, C.; et al. Phosphodiesterase 7 inhibitor reduced cognitive impairment and pathological hallmarks in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [Green Version]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA 2014, 311, 33–44. [Google Scholar] [CrossRef]
- Ibrahim, N.F.; Yanagisawa, D.; Durani, L.W.; Hamezah, H.S.; Damanhuri, H.A.; Wan Ngah, W.Z.; Tsuji, M.; Kiuchi, Y.; Ono, K.; Tooyama, I. Tocotrienol-Rich Fraction Modulates Amyloid Pathology and Improves Cognitive Function in AbetaPP/PS1 Mice. J. Alzheimers Dis. 2017, 55, 597–612. [Google Scholar] [CrossRef]




| OS Markers | AD | PSP | PiD | CBD | FTD | FTDP-17 | FTLD | PD |
|---|---|---|---|---|---|---|---|---|
| SOD1 | ↓ [27] ↑ [63] | ↑ [11] | ↑ [64] | ↑ [63] | ||||
| SOD2 | ↓ [27] ↑ [65] | ↑ [11] | ↓ [66] | ↑ [64] | ↑ [67] | |||
| Cu/Zn-SOD | ↓ [27] ↑ [68] | ↑ [11] | ↑ [63] | |||||
| GSH | ↓ [27] | ↑ [69] ↓ [70] | ↓ [64] | ↓ [69] | ||||
| GST | ↓ [71] | ↓ [11] | ||||||
| Catalase | ↓ [72] | ↓ [73] | ||||||
| GPx | ↓ [72] ↑ [74] | ↑ [75] | ↑ [11] ↓ [11] | ↑ [76] ↓ [67] | ||||
| Lipid peroxidase | ↑ [27] | ↑ [77] | ↑ [78] | ↑ [79] | ||||
| MDA | ↑ [27] | ↑ [80] | ↑ [64] | ↑ [81] | ||||
| 4-HNE | ↑ [27] | ↑ [75] | ↑ [81] | ↑ [64] | ↑ [82] | |||
| Vitamin A | ↓ [83] | |||||||
| Vitamin C | ↓ [84] | ↓ [73] | ||||||
| Vitamin E | ↓ [85] | ↓ [73] | ||||||
| HO-1 | ↑ [86] | ↑ [86] | ↑ [86] | ↑ [86] | ↑ [78] | ↑ [87] | ||
| Protein carbonyl | ↑ [27] | ↑ [78] | ↑ [63] | |||||
| 3-nitrotyrosine | ↑ [27] | ↑ [88] | ||||||
| 8-OHG | ↑ [27] | ↑ [89] | ||||||
| 8-OHdG | ↑ [27] | ↑ [89] | ||||||
| F2-IsoPs | ↑ [27] | |||||||
| Hcy | ↑ [90] | |||||||
| COX | ↑ [91] | |||||||
| PDHC | ↓ [92] | |||||||
| KGDHC | ↓ [93] |
| ROS | Fluorescent Probe | Reaction | Examples |
|---|---|---|---|
| Hydrogen peroxide | H2O2-induced oxidation of arylboronate ester to phenols. |  |  (Ref: 1 [98], 2 [99]) |
| Superoxide | Nucleophilic substitution reaction of superoxide with the probes where the leaving group is phosphinate group [104], triflate group [105], 2,4-dinitrobenzenesulfonyl [106], nitro-ethers [107] and the related groups. |  |  (Ref: 3 [128],4 [108]) |
| Hydroxyl radical | Hydroxyl radical-mediated oxidation of leuco forms of fluorescent dyes and nitroxide moiety. |  |  (Ref: 5 [112], 6 [129]) |
| Singlet oxygen | The fluorescent probes for singlet oxygen have been developed based on the [2 + 4] cycloaddition where singlet oxygen acts as a strong dienophile [109,114,115]. |  |  (Ref: 7 [115]) |
| Peroxynitrite | Peroxynitrite-mediated oxidation of chalcogenides (S, Se, and Te), boronic acids or boronates, hydrazides, cleavage of C–C double bonds and oxidative N-dearylation. |  |  (Ref: 8 [122]) |
| Nitric oxide | NO-mediated reaction of O-diaminophenyl group with NO to generate the triazole. |  |  (Ref: 9 [130]) |
| Hypochlorite | Hypochlorite mediated spiro-ring opening of the xanthene probes and oxidation of catechol to benzoquinone [122]. |  |  (Ref: 10 [117]) |
| Therapeutic Approaches | Chemical Agents | Treating Route and Doses | Experimental Model | Reference |
|---|---|---|---|---|
| Antioxidant pathway | Curcumin | 10 μg/mL for 1 h | Aβ treated PC12 cell | [131] |
| Methylene blue | 4 mg/kg in diet | TauP301S mouse | [132] | |
| CoQ10 | 0.5% in diet | TauP301S mouse | [133] | |
| Paraquant | 30 mM in diet for 48 h | TauR406W drosophila | [66] | |
| Sulforaphane | 5 mg/kg twice a week by intraperitoneally | C57Bl/66-OHDA mouse | [134] | |
| BR 297 | 500 nM for 24 h | APP treated SH-SY5Y cell | [145] | |
| S14 | 5 mg/Kg/daily for 4 weeks by intraperitoneally | Tg2576APP/PS1 AD mouse | [146] | |
| Resveratrol | 500–1000 mg/daily for 26 months by orally | AD patients | [147] | |
| Catalase | EUK-207 | 3.41 mM/day for 28 days by micro-osmotic pump | C57BL/6/129S-3xTg-AD mouse | [136] |
| Vitamin | Ascorbic acid | 250–500 μM for 24 h | Neurons form Tau151–391 rat | [138] |
| α-tocopherol | 0.5–1.5 mM in diet for 10 days | tauR406W drosophila | [66] | |
| 49 IU/Kg in diet | B6D2/F1-tau44 mouse | [139] | ||
| 2000 IU/day for 6 months | AD patient | [148] | ||
| Tocotrienol | 5 mg/Kg/day for 15 months by orally | APPswe/PS1dE9 mouse | [149] | |
| Metal chelator | Clioquinol | 30 mg/kg/day for 5 weeks by orally | TgCRND8-AD mouse | [141] |
| PBT2 | 250 mg/day for 12 weeks by orally | AD patient | [142] | |
| CuIIGTSM | 10 mg/kg/daily by orally | APP/PS1 AD mouse | [143] | |
| Desferrioxamine | 125 mg twice daily/5 days per week for 24 months by intramuscularly | AD patient | [144] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, M.M.; Murale, D.P.; Kim, Y.K.; Lee, J.-S. Crosstalk between Oxidative Stress and Tauopathy. Int. J. Mol. Sci. 2019, 20, 1959. https://doi.org/10.3390/ijms20081959
Haque MM, Murale DP, Kim YK, Lee J-S. Crosstalk between Oxidative Stress and Tauopathy. International Journal of Molecular Sciences. 2019; 20(8):1959. https://doi.org/10.3390/ijms20081959
Chicago/Turabian StyleHaque, Md. Mamunul, Dhiraj P. Murale, Yun Kyung Kim, and Jun-Seok Lee. 2019. "Crosstalk between Oxidative Stress and Tauopathy" International Journal of Molecular Sciences 20, no. 8: 1959. https://doi.org/10.3390/ijms20081959