Fomes fomentarius Ethanol Extract Exerts Inhibition of Cell Growth and Motility Induction of Apoptosis via Targeting AKT in Human Breast Cancer MDA-MB-231 Cells
Abstract
:1. Introduction
2. Results
2.1. F. fomentarius Ethanol Extract (FFE) Exerts Anti-Proliferative and Cytotoxic Effects in MDA-MB-231 Cells
2.2. FFE Increases S-Phase Arrest and Apoptosis Rates and Regulates Cell Cycle- and Apoptosis-Related Proteins
2.3. FFE Inhibits Cell Migration
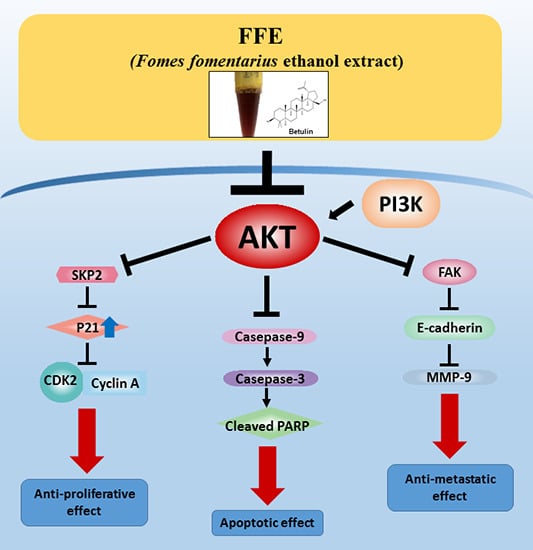
2.4. AKT Mediates FFE-Induced Suppression of Cell Proliferation and Migration
2.5. Betulin and Daphnetin in FFE Decrease Phosphorylated AKT in MDA-MB-231 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Fomes fomentarius Ethanol Extract Preparation
4.3. Cytotoxicity Assay
4.4. Western Blotting
4.5. Fluoresecence-activated cell sorting (FACS) Analysis
4.6. Crystal Violet Staining Assay, Colony Formation Assay, Cell Growth Assay
4.7. Wound Healing Assay
4.8. Proliferation Assay
4.9. HPLC Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FFE | fomentarius ethanol extract |
| MMP-9 | matrix metalloproteinase-9 |
| p-AKT | phosphorylation of AKT |
| CDK2 | cyclin-dependent kinase 2 |
| SKP2 | S-phase kinase-associated protein 2 |
| BCL-2 | B-cell lymphoma 2 |
| HER-2 | human epidermal growth factor receptor 2 |
| PTEN | phosphatase and tensin homolog |
| Wort | wortmannin |
| HPLC | high-performance liquid chromatography |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| O.D. | optical density; BrdU, bromodeoxyuridine |
References
- Breast Cancer. Available online: https:www.breastcancer.org (accessed on 30 July 2001).
- Costa, R.L.; Han, H.S.; Gradishar, W.J. Targeting the PI3K/AKT/MTOR pathway in triple-negative breast cancer: A review. Breast Cancer Res. Treat. 2018, 169, 397–406. [Google Scholar] [PubMed]
- Paplomata, E.; O’Regan, R. The PI3K/AKT/MTOR pathway in breast cancer: Targets, trials and biomarkers. Ther. Adv. Med. Oncol. 2014, 6, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, G.I.; Dicitore, A.; Gaudenzi, G.; Caraglia, M.; Persani, L.; Vitale, G. PI3K/AKT/MTOR signaling in medullary thyroid cancer: A promising molecular target for cancer therapy. Endocrine 2015, 48, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.Q.; Li, J.; Li, S.Q.; Cao, Y.L. Inhibition of phosphoinositide 3-kinase delta attenuates experimental autoimmune encephalomyelitis in mice. Int. J. Clin. Exp. Med. 2015, 8, 20645–20651. [Google Scholar] [PubMed]
- Liu, T.; Yacoub, R.; Taliaferro-Smith, L.D.; Sun, S.Y.; Graham, T.R.; Dolan, R.; Lobo, C.; Tighiouart, M.; Yang, L.; Adams, A.; et al. Combinatorial effects of lapatinib and rapamycin in triple-negative breast cancer cells. Mol. Cancer Ther. 2011, 10, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Cossu-Rocca, P.; Orru, S.; Muroni, M.R.; Sanges, F.; Sotgiu, G.; Ena, S.; Pira, G.; Murgia, L.; Manca, A.; Uras, M.G.; et al. Analysis of PIK3CA mutations and activation pathways in triple negative breast cancer. PLoS ONE 2015, 10, e0141763. [Google Scholar]
- Ooms, L.M.; Binge, L.C.; Davies, E.M.; Rahman, P.; Conway, J.R.; Gurung, R.; Ferguson, D.T.; Papa, A.; Fedele, C.G.; Vieusseux, J.L.; et al. The inositol polyphosphate 5-phosphatase PIPP regulates AKT1-dependent breast cancer growth and metastasis. Cancer Cell 2015, 28, 155–169. [Google Scholar] [PubMed]
- Paplomata, E.; O’Regan, R. New and emerging treatments for estrogen receptor-positive breast cancer: Focus on everolimus. Ther. Clin. Risk Manag. 2013, 9, 27–36. [Google Scholar] [PubMed]
- Nahta, R. Pharmacological strategies to overcome HER2 cross-talk and trastuzumab resistance. Curr. Med. Chem. 2012, 19, 1065–1075. [Google Scholar] [PubMed]
- Dresch, P.; MN, D.A.; Rosam, K.; Grienke, U.; Rollinger, J.M.; Peintner, U. Fungal strain matters: Colony growth and bioactivity of the european medicinal polypores fomes fomentarius, fomitopsis pinicola and piptoporus betulinus. AMB Express 2015, 5, 4. [Google Scholar] [PubMed]
- Kim, S.H.; Jakhar, R.; Kang, S.C. Apoptotic properties of polysaccharide isolated from fruiting bodies of medicinal mushroom fomes fomentarius in human lung carcinoma cell line. Saudi J. Biol. Sci. 2015, 22, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Xiong, J.; Zhai, W.Z.; Cao, L.; Zhang, S.P.; Tang, Y.; Wang, J.; Su, J.J.; Yang, G.X.; Zhao, Y.; et al. Fomentarols a-d, sterols from the polypore macrofungus fomes fomentarius. Phytochemistry 2013, 92, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, Z.; Chen, S.F.; Li, Y.Q. Optimization for the production of exopolysaccharide from fomes fomentarius in submerged culture and its antitumor effect in vitro. Bioresour. Technol. 2008, 99, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S. Effects of fomes fomentarius supplementation on a17ntioxidant enzyme activities, blood glucose, and lipid profile in streptozotocin-induced diabetic rats. Nutr. Res. 2005, 25, 187–195. [Google Scholar] [CrossRef]
- Park, Y.-M.; Kim, I.-T.; Park, H.-J.; Choi, J.-W.; Park, K.-Y.; Lee, J.-D.; Nam, B.-H.; Kim, D.-G.; Lee, J.-Y.; Lee, K.-T. Anti-inflammatory and anti-nociceptive effects of the methanol extract of fomes fomentarius. Biol. Pharm. Bull. 2004, 27, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Committee, S.D.W.; Council, N.R. Drinking Water and Health; National Academies Press: Washington, DC, USA, 1977; Volume 1. [Google Scholar]
- Huang, T.; Du, D.; Chen, Y.; Yuan, B.; Ju, X.; Feng, Y.; Wang, L.; Jiang, J. Chemical constituents and antitumor activity of fruiting body of fomes fomentarius. Mycosystema 2012, 5, 775–783. [Google Scholar]
- Grienke, U.; Zoll, M.; Peintner, U.; Rollinger, J.M. European medicinal polypores—A modern view on traditional uses. J. Ethnopharmacol. 2014, 154, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Mbaveng, A.T.; Fotso, G.W.; Ngnintedo, D.; Kuete, V.; Ngadjui, B.T.; Keumedjio, F.; Andrae-Marobela, K.; Efferth, T. Cytotoxicity of epunctanone and four other phytochemicals isolated from the medicinal plants garcinia epunctata and ptycholobium contortum towards multi-factorial drug resistant cancer cells. Phytomed. Int. J. Phytother. Phytopharm. 2018, 48, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Arcaro, A.; Guerreiro, A.S. The phosphoinositide 3-kinase pathway in human cancer: Genetic alterations and therapeutic implications. Curr. Genom. 2007, 8, 271–306. [Google Scholar] [CrossRef] [PubMed]
- Ghayad, S.E.; Cohen, P.A. Inhibitors of the pi3k/akt/mtor pathway: New hope for breast cancer patients. Recent Pat. Anti-Cancer Drug Discov. 2010, 5, 29–57. [Google Scholar] [CrossRef]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/AKT/MTOR signaling in cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.G.; Castillo-Martin, M.; Bonal, D.M.; Jia, A.J.; Rybicki, B.A.; Christiano, A.M.; Cordon-Cardo, C. PI3K/AKT pathway regulates e-cadherin and desmoglein 2 in aggressive prostate cancer. Cancer Med. 2015, 4, 1258–1271. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiu, Z.; Li, F.; Wang, C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol. Lett. 2017, 14, 5865–5870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossig, L.; Jadidi, A.S.; Urbich, C.; Badorff, C.; Zeiher, A.M.; Dimmeler, S. Akt-dependent phosphorylation of p21(cip1) regulates pcna binding and proliferation of endothelial cells. Mol. Cell. Biol. 2001, 21, 5644–5657. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Kawabe, T.; Ohara, H.; Ducommun, B.; Itoh, M.; Okamoto, T. Involvement of the interaction between p21 and proliferating cell nuclear antigen for the maintenance of G2/M arrest after DNA damage. J. Biol. Chem. 2001, 276, 42971–42977. [Google Scholar] [CrossRef] [PubMed]
- Shnyreva, A.V.; Shnyreva, A.; Espinoza, C.; Padrón, J.M.; Trigos, Á. Antiproliferative activity and cytotoxicity of some medicinal wood-destroying fungi. Int. J. Med. Mushrooms 2018, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Yao, W.; Zheng, J.; Ding, W.; Wang, Y.; Zhang, T.; Zhu, L.; Zhou, F. A derivative of betulinic acid protects human retinal pigment epithelial (rpe) cells from cobalt chloride-induced acute hypoxic stress. Exp. Eye Res. 2019, 180, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Cháirez-Ramírez, M.; Moreno-Jiménez, M.; González-Laredo, R.; Gallegos-Infante, J.; Rocha-Guzmán, N.E. Lupane-type triterpenes and their anti-cancer activities against most common malignant tumors: A review. EXCLI J. 2016, 15, 758. [Google Scholar] [PubMed]
- Härmä, V.; Haavikko, R.; Virtanen, J.; Ahonen, I.; Schukov, H.-P.; Alakurtti, S.; Purev, E.; Rischer, H.; Yli-Kauhaluoma, J.; Moreira, V.M. Optimization of invasion-specific effects of betulin derivatives on prostate cancer cells through lead development. PLoS ONE 2015, 10, e0126111. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.J.; Hsu, Y.C.; Chen, S.P.; Fu, C.L.; Yu, J.C.; Chang, F.W.; Chen, Y.H.; Liu, J.M.; Ho, J.Y.; Yu, C.P. The triterpenoids of hibiscus syriacus induce apoptosis and inhibit cell migration in breast cancer cells. BMC Complement. Altern. Med. 2015, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Ci, X.; Zhou, J.; Lv, H.; Yu, Q.; Peng, L.; Hua, S. Betulin exhibits anti-inflammatory activity in lps-stimulated macrophages and endotoxin-shocked mice through an AMPK/AKT/NRF2-dependent mechanism. Cell Death Dis. 2017, 8, e2798. [Google Scholar] [PubMed]
- Lee, M.-S.; Cho, S.-M.; Kim, J.-S.; Kim, S.-H.; Lee, H.-J. Ethanol extract of the pinus koraiensis leaves anti-obesity and hypolipidemic effects by activating the ampk signaling. Nutr. Food Sci. 2016, 16, 51. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-O.; Lee, M.-H.; Lee, K.-R.; Lee, E.-O.; Lee, H.-J. Fomes fomentarius Ethanol Extract Exerts Inhibition of Cell Growth and Motility Induction of Apoptosis via Targeting AKT in Human Breast Cancer MDA-MB-231 Cells. Int. J. Mol. Sci. 2019, 20, 1147. https://doi.org/10.3390/ijms20051147
Lee S-O, Lee M-H, Lee K-R, Lee E-O, Lee H-J. Fomes fomentarius Ethanol Extract Exerts Inhibition of Cell Growth and Motility Induction of Apoptosis via Targeting AKT in Human Breast Cancer MDA-MB-231 Cells. International Journal of Molecular Sciences. 2019; 20(5):1147. https://doi.org/10.3390/ijms20051147
Chicago/Turabian StyleLee, Seon-OK, Min-Ho Lee, Kyung-Ran Lee, Eun-Ok Lee, and Hyo-Jeong Lee. 2019. "Fomes fomentarius Ethanol Extract Exerts Inhibition of Cell Growth and Motility Induction of Apoptosis via Targeting AKT in Human Breast Cancer MDA-MB-231 Cells" International Journal of Molecular Sciences 20, no. 5: 1147. https://doi.org/10.3390/ijms20051147