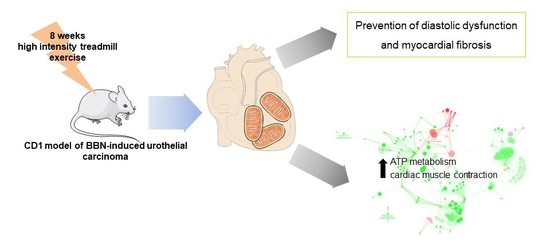
Exercise Training Impacts Cardiac Mitochondrial Proteome Remodeling in Murine Urothelial Carcinoma
Abstract
:1. Introduction
2. Results
2.1. Characterization of Mice Response to BBN Exposure and/or Endurance Training
2.2. Characterization of Cardiac Function and Morphometry
2.3. Proteomic Profiling of Cardiac Mitochondria
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Hemodynamic Evaluation
4.3. Blood Tests
4.4. Histological Analysis of Urinary Bladder and Cardiac Muscle
4.5. Mitochondria Isolation
4.6. Determination of ATP Synthase Activity
4.7. Determination of Citrate Synthase (CS) Activity
4.8. Immunoblotting Analysis
4.9. GeLC-MS/MS Analysis of Isolated Mitochondria
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Aapro, M.; Arends, J.; Bozzetti, F.; Fearon, K.; Grunberg, S.M.; Herrstedt, J.; Hopkinson, J.; Jacquelin-Ravel, N.; Jatoi, A.; Kaasa, S.; et al. Early recognition of malnutrition and cachexia in the cancer patient: A position paper of a European School of Oncology Task Force. Ann. Oncol 2014, 25, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J.M.; Busquets, S.; Stemmler, B.; Lopez-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.C.; Oreto, L.; Qamar, R.; Paterick, T.E.; Carerj, S.; Khandheria, B.K. Cardioncology: State of the heart. Int. J. Cardiol. 2013, 168, 680–687. [Google Scholar] [CrossRef]
- Schunemann, M.; Anker, S.D.; Rauchhaus, M. Cancer fatigue syndrome reflects clinically non-overt heart failure: An approach towards onco-cardiology. Nat. Clin. Pract. Oncol. 2008, 5, 632–633. [Google Scholar] [CrossRef] [PubMed]
- Padrao, A.I.; Moreira-Goncalves, D.; Oliveira, P.A.; Teixeira, C.; Faustino-Rocha, A.I.; Helguero, L.; Vitorino, R.; Santos, L.L.; Amado, F.; Duarte, J.A.; et al. Endurance training prevents TWEAK but not myostatin-mediated cardiac remodelling in cancer cachexia. Arch. Biochem. Biophys. 2015, 567, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Asp, M.L.; Nishijima, Y.; Belury, M.A. Evidence for cardiac atrophic remodeling in cancer-induced cachexia in mice. Int. J. Oncol. 2011, 39, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Bagnato, A.; Battistini, B.; Nisen, P. The endothelin axis: Emerging role in cancer. Nat. Rev. Cancer 2003, 3, 110–116. [Google Scholar] [CrossRef]
- Pavo, N.; Raderer, M.; Hulsmann, M.; Neuhold, S.; Adlbrecht, C.; Strunk, G.; Goliasch, G.; Gisslinger, H.; Steger, G.G.; Hejna, M.; et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart 2015, 101, 1874–1880. [Google Scholar] [CrossRef] [Green Version]
- Ohsaki, Y.; Gross, A.J.; Le, P.T.; Oie, H.; Johnson, B.E. Human small cell lung cancer cells produce brain natriuretic peptide. Oncology 1999, 56, 155–159. [Google Scholar] [CrossRef]
- Danese, E.; Montagnana, M.; Giudici, S.; Aloe, R.; Franchi, M.; Guidi, G.C.; Lippi, G. Highly-sensitive troponin I is increased in patients with gynecological cancers. Clin. Biochem. 2013, 46, 1135–1138. [Google Scholar] [CrossRef]
- Gielen, S.; Laughlin, M.H.; O’Conner, C.; Duncker, D.J. Exercise Training in Patients with Heart Disease: Review of Beneficial Effects and Clinical Recommendations. Prog. Cardiovasc. Dis. 2015, 57, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Moreira-Goncalves, D.; Azevedo, A.L.; Duarte, J.A.; Amado, F.; Vitorino, R. Unraveling the exercise-related proteome signature in heart. Basic Res. Cardiol. 2015, 110, 454. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.M.M.; Ferreira, R.M.P.; Moreira-Gonçalves, D. Exercise Training as Therapy for Cancer-Induced Cardiac Cachexia. Trends Mol. Med. 2018, 24, 709–727. [Google Scholar] [CrossRef] [PubMed]
- Padrão, A.I.; Nogueira-Ferreira, R.; Vitorino, R.; Carvalho, D.; Correia, C.; Neuparth, M.J.; Pires, M.J.; Faustino-Rocha, A.I.; Santos, L.L.; Oliveira, P.A.; et al. Exercise training protects against cancer-induced cardiac remodeling in an animal model of urothelial carcinoma. Arch. Biochem. Biophys. 2018, 645, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Vitorino, R.; Padrao, A.I.; Espadas, G.; Mancuso, F.M.; Moreira-Goncalves, D.; Castro-Sousa, G.; Henriques-Coelho, T.; Oliveira, P.A.; Barros, A.S.; et al. Lifelong exercise training modulates cardiac mitochondrial phosphoproteome in rats. J. Proteome Res. 2014, 13, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, E.; Deliu, E.; Sporici, R.A.; Brailoiu, G.C. Irisin evokes bradycardia by activating cardiac-projecting neurons of nucleus ambiguus. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- X’Avia Chan, C.Y.; Wang, D.; Cadeiras, M.; Deng, M.C.; Ping, P. S-nitrosylation of TRIM72 mends the broken heart: A molecular modifier-mediated cardioprotection. J. Mol. Cell Cardiol. 2014, 72, 292–295. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, P.A.; Colaco, A.; De la Cruz, P.L.; Lopes, C. Experimental bladder carcinogenesis-rodent models. Exp. Oncol. 2006, 28, 2–11. [Google Scholar]
- Padrao, A.I.; Oliveira, P.; Vitorino, R.; Colaco, B.; Pires, M.J.; Marquez, M.; Castellanos, E.; Neuparth, M.J.; Teixeira, C.; Costa, C.; et al. Bladder cancer-induced skeletal muscle wasting: Disclosing the role of mitochondria plasticity. Int. J. Biochem. Cell Biol. 2013, 45, 1399–1409. [Google Scholar] [CrossRef]
- Xu, H.; Crawford, D.; Hutchinson, K.R.; Youtz, D.J.; Lucchesi, P.A.; Velten, M.; McCarthy, D.O.; Wold, L.E. Myocardial dysfunction in an animal model of cancer cachexia. Life Sci. 2011, 88, 406–410. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Nishijima, Y.; Asp, M.L.; Stout, M.B.; Reiser, P.J.; Belury, M.A. Cardiac alterations in cancer-induced cachexia in mice. Int. J. Oncol. 2010, 37, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.C.; Lin, J.L.C.; Erives, A.J.; Lin, C.I.; Lin, J.J.C. New Insights into the Roles of Xin Repeat-Containing Proteins in Cardiac Development, Function, and Disease. Int. Rev. Cell Mol. Biol. 2014, 310, 89–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Lin, J.L.; Wu, K.H.; Wang, D.Z.; Reiter, R.S.; Sinn, H.W.; Lin, C.I.; Lin, C.J. Xin proteins and intercalated disc maturation, signaling and diseases. Front. Biosci. 2012, 17, 2566–2593. [Google Scholar] [CrossRef]
- Kebir, S.; Orfanos, Z.; Schuld, J.; Linhart, M.; Lamberz, C.; van der Ven, P.F.; Schrickel, J.; Kirfel, G.; Furst, D.O.; Meyer, R. Sarcomeric lesions and remodeling proximal to intercalated disks in overload-induced cardiac hypertrophy. Exp. Cell Res. 2016, 348, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Rocha, H.; Almeida, V.; Padrao, A.I.; Santa, C.; Vilarinho, L.; Amado, F.; Vitorino, R. Mitochondria proteome profiling: A comparative analysis between gel- and gel-free approaches. Talanta 2013, 115, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Rocha, H.; Ferreira, R.; Carvalho, J.; Vitorino, R.; Santa, C.; Lopes, L.; Gregersen, N.; Vilarinho, L.; Amado, F. Characterization of mitochondrial proteome in a severe case of ETF-QO deficiency. J. Proteomics 2011, 75, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Mann, N.; Rosenzweig, A. Can exercise teach us how to treat heart disease? Circulation 2012, 126, 2625–2635. [Google Scholar] [CrossRef]
- Ferreira, R.; Nogueira-Ferreira, R.; Trindade, F.; Vitorino, R.; Powers, S.K.; Moreira-Goncalves, D. Sugar or fat: The metabolic choice of the trained heart. Metabolism 2018, 87, 98–104. [Google Scholar] [CrossRef]
- Lai, L.; Leone, T.C.; Keller, M.P.; Martin, O.J.; Broman, A.T.; Nigro, J.; Kapoor, K.; Koves, T.R.; Stevens, R.; Ilkayeva, O.R.; et al. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: A multisystems approach. Circ. Heart Fail. 2014, 7, 1022–1031. [Google Scholar] [CrossRef]
- Kavazis, A.N.; Alvarez, S.; Talbert, E.; Lee, Y.; Powers, S.K. Exercise training induces a cardioprotective phenotype and alterations in cardiac subsarcolemmal and intermyofibrillar mitochondrial proteins. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H144–H152. [Google Scholar] [CrossRef] [Green Version]
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Med. Sci. Sports Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasconcelos-Nobrega, C.; Pinto-Leite, R.; Arantes-Rodrigues, R.; Ferreira, R.; Brochado, P.; Cardoso, M.L.; Palmeira, C.; Salvador, A.; Guedes-Teixeira, C.I.; Colaco, A.; et al. In vivo and in vitro effects of RAD001 on bladder cancer. Urol. Oncol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Kemi, O.J.; Loennechen, J.P.; Wisloff, U.; Ellingsen, O. Intensity-controlled treadmill running in mice: Cardiac and skeletal muscle hypertrophy. J. Appl. Physiol. 2002, 93, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Goncalves, D.; Henriques-Coelho, T.; Fonseca, H.; Ferreira, R.; Padrao, A.I.; Santa, C.; Vieira, S.; Silva, A.F.; Amado, F.; Leite-Moreira, A.; et al. Intermittent cardiac overload results in adaptive hypertrophy and provides protection against left ventricular acute pressure overload insult. J. Physiol. 2015, 593, 3885–3897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forbes, D.B.H.; Kostomitsopulos, N.; Moore, G.; Perretta, G. Euroguide: On the Accommodation and Care of Animals Used for Experimental and Other Scientific Purposes. FELASA: London, UK. Available online: http://www.felasa.eu/about-us/library/ (accessed on 29 December 2018).
- Oliveira, P.A.; Arantes-Rodrigues, R.; Sousa-Diniz, C.; Colaco, A.; Lourenco, L.; De La Cruz, L.F.; Da Silva, V.M.; Afonso, J.; Lopes, C.; Santos, L. The effects of sirolimus on urothelial lesions chemically induced in ICR mice by BBN. Anticancer Res. 2009, 29, 3221–3226. [Google Scholar] [PubMed]
- Padrao, A.I.; Ferreira, R.; Vitorino, R.; Alves, R.M.; Figueiredo, P.; Duarte, J.A.; Amado, F. Effect of lifestyle on age-related mitochondrial protein oxidation in mice cardiac muscle. Eur. J. Appl. Physiol. 2012, 112, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.; Morin, C.; Urien, S.; Tillement, J.P.; Bruguerolle, B. Tacrolimus and sirolimus decrease oxidative phosphorylation of isolated rat kidney mitochondria. Br. J. Pharmacol. 2003, 138, 369–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coore, H.G.; Denton, R.M.; Martin, B.R.; Randle, P.J. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem. J. 1971, 125, 115–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Ishihama, Y.; Oda, Y.; Tabata, T.; Sato, T.; Nagasu, T.; Rappsilber, J.; Mann, M. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell Proteomics 2005, 4, 1265–1272. [Google Scholar] [CrossRef] [PubMed]






| Experimental Group | Body Weight (g) | Heart Weight (g) | Gastrocnemius Mass (g) | Heart-to-Body Weight (mg/g) |
|---|---|---|---|---|
| CONT+Sed | 39.23 ± 2.92 | 0.20 ± 0.03 | 0.40 ± 0.02 | 5.24 ± 0.94 |
| CONT+Ex | 39.39 ± 3.58 | 0.22 ± 0.04 | 0.35 ± 0.06 | 5.61 ± 0.98 |
| BBN+Sed | 40.69 ± 2.41 | 0.21 ± 0.03 | 0.42 ± 0.04 | 5.18 ± 0.50 |
| BBN+Ex | 40.93 ± 2.59 | 0.21 ± 0.04 | 0.39 ± 0.04 | 5.22 ± 1.12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, R.; Neuparth, M.J.; Nogueira-Ferreira, R.; Magalhães, S.; Aroso, M.; Bovolini, J.A.; Lara Santos, L.; Oliveira, P.; Vitorino, R.; Moreira-Gonçalves, D. Exercise Training Impacts Cardiac Mitochondrial Proteome Remodeling in Murine Urothelial Carcinoma. Int. J. Mol. Sci. 2019, 20, 127. https://doi.org/10.3390/ijms20010127
Ferreira R, Neuparth MJ, Nogueira-Ferreira R, Magalhães S, Aroso M, Bovolini JA, Lara Santos L, Oliveira P, Vitorino R, Moreira-Gonçalves D. Exercise Training Impacts Cardiac Mitochondrial Proteome Remodeling in Murine Urothelial Carcinoma. International Journal of Molecular Sciences. 2019; 20(1):127. https://doi.org/10.3390/ijms20010127
Chicago/Turabian StyleFerreira, Rita, Maria João Neuparth, Rita Nogueira-Ferreira, Sandra Magalhães, Miguel Aroso, José António Bovolini, Lúcio Lara Santos, Paula Oliveira, Rui Vitorino, and Daniel Moreira-Gonçalves. 2019. "Exercise Training Impacts Cardiac Mitochondrial Proteome Remodeling in Murine Urothelial Carcinoma" International Journal of Molecular Sciences 20, no. 1: 127. https://doi.org/10.3390/ijms20010127