Cardiovascular Benefits of Exercise Training in Postmenopausal Hypertension
Abstract
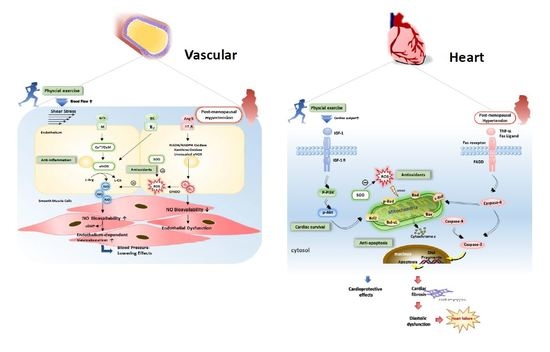
:1. Introduction
1.1. Relation between Body Mass Index, Exercise Training
1.2. Antihypertensive Effects of Exercise Training by Cardiovascular Autonomic Regulation
1.3. Effect of Exercise Training on Lipid Profiles
1.4. Antioxidative or Anti-Inflammatory Effects of Exercise Training
1.5. Antihypertensive Effects of Exercise Training by Improved NO Bioavailability and Vasorelaxation
1.6. Cardiac Remodeling, Antifibrosis, or Antiapoptotic Effect of Exercise Training
1.7. Aerobic Training vs. Resistance Training for Postmenopausal Hypertension
2. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Farahmand, M.; Ramezani Tehrani, F.; Bahri Khomami, M.; Noroozzadeh, M.; Azizi, F. Surgical menopause versus natural menopause and cardio-metabolic disturbances: A 12-year population-based cohort study. J. Endocrinol. Investig. 2015, 38, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.Z.; Sangawan, V.; Das, A.; Pandey, A.K. Assessment of cardiovascular risk in natural and surgical menopause. J. Endocrinol. Metab. 2018, 22, 223–228. [Google Scholar]
- Staessen, J.A.; Celis, H.; Fagard, R. The epidemiology of the association between hypertension and menopause. J. Hum. Hypertens. 1998, 12, 587–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, R.; Grimaldi, T.; Origliani, G.; Fantini, G.; Coppi, F.; Modena, M.G. Menopause and cardiovascular risk. Pathophysiol. Haemost. Thromb. 2002, 32, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, A.; Fleg, J.L. Hypertension in postmenopausal women as a medical and public health problem. High.Blood Press. Cardiovasc. Prev. 2003, 10, 51–55. [Google Scholar] [CrossRef]
- Guetta, V.; Cannon, R.O. Cardiovascular effects of estrogen and lipid-lowering therapies in postmenopausal women. Circulation 1996, 93, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, C.; Baron, J.A.; Correia, N.; Bergstrom, R.; Adami, H.O.; Persson, I. Breast-cancer risk following long-term oestrogen- and oestrogen-progestin-replacement therapy. Int. J. Cancer 1999, 81, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.J.; Phipps, W.R.; Thomas, W.; Schmitz, K.H.; Kurzer, M.S. The effects of aerobic exercise on estrogen metabolism in healthy premenopausal women. Cancer Epidemiol. Prev. Biomark. 2013, 22, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Myers, J. Exercise and cardiovascular health. Circulation 2003, 107, e2–e5. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Thomas, R.J.; Squires, R.W.; Allison, T.G.; Milani, R.V. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clinic Proc. 2009, 84, 373–383. [Google Scholar] [CrossRef]
- Fagard, R.H. Exercise therapy in hypertensive cardiovascular disease. Progr. Cardiovasc. Dis. 2011, 53, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Zanesco, A.; Zaros, P.R. Physical exercise and menopause. Rev. Bras. Ginecol. Obstet. 2009, 31, 254–261. [Google Scholar] [PubMed]
- Carr, M.C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Claudio, E.R.; Almeida, S.A.; Mengal, V.; Brasil, G.A.; Santuzzi, C.H.; Tiradentes, R.V.; Gouvea, S.A.; Bissoli, N.S.; Santos, R.L.; Abreu, G.R. Swimming training prevents coronary endothelial dysfunction in ovariectomized spontaneously hypertensive rats. Braz. J. Med. Biol. Res. 2017, 50, e5495. [Google Scholar] [CrossRef] [PubMed]
- Sanches, I.C.; de Oliveira Brito, J.; Candido, G.O.; da Silva Dias, D.; Jorge, L.; Irigoyen, M.C.; De Angelis, K. Cardiometabolic benefits of exercise training in an experimental model of metabolic syndrome and menopause. Menopause 2012, 19, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.M.; Nascimento, F.A.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Exercise training attenuates cardiovascular adverse remodeling in adult ovariectomized spontaneously hypertensive rats. Menopause 2006, 13, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, A.; Hamilton, D.J.; Deng, T. Epicardial fat in the maintenance of cardiovascular health. Debakey Cardiovasc. J. 2017, 13, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, N.; Moreno-Villegas, Z.; Gonzalez-Bris, A.; Egido, J.; Lorenzo, O. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Park, K.S.; McCormick, J.B. Effects of exercise training on fat loss and lean mass gain in mexican-american and korean premenopausal women. Int. J. Endocrinol. 2017, 2017, 5465869. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Kim, J.W.; Kim, D.Y. Effects of yoga exercise on serum adiponectin and metabolic syndrome factors in obese postmenopausal women. Menopause 2012, 19, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Khalid, T.; Nesreen, E.; Ramadhan, O. Effects of exercise training on postmenopausal hypertension: implications on nitric oxide levels. Med. J. Malays. 2013, 68, 459–464. [Google Scholar]
- Jarrete, A.P.; Novais, I.P.; Nunes, H.A.; Puga, G.M.; Delbin, M.A.; Zanesco, A. Influence of aerobic exercise training on cardiovascular and endocrine-inflammatory biomarkers in hypertensive postmenopausal women. J. Clin. Transl. Endocrinol. 2014, 1, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Son, W.M.; Sung, K.D.; Cho, J.M.; Park, S.Y. Combined exercise reduces arterial stiffness, blood pressure, and blood markers for cardiovascular risk in postmenopausal women with hypertension. Menopause 2017, 24, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Maslow, A.L.; Sui, X.; Colabianchi, N.; Hussey, J.; Blair, S.N. Muscular strength and incident hypertension in normotensive and prehypertensive men. Med. Sci. Sports Exerc. 2010, 42, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Park, S.Y.; Seo, D.Y.; Sanchez-Gonzalez, M.A.; Baek, Y.H. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause 2011, 18, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Latosik, E.; Zubrzycki, I.Z.; Ossowski, Z.; Bojke, O.; Clarke, A.; Wiacek, M.; Trabka, B. Physiological responses associated with nordic-walking training in systolic hypertensive postmenopausal women. J. Hum. Kinet. 2014, 43, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Staffileno, B.A.; Braun, L.T.; Rosenson, R.S. The accumulative effects of physical activity in hypertensive post-menopausal women. J. Cardiovasc. Risk 2001, 8, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Arca, E.A.; Martinelli, B.; Martin, L.C.; Waisberg, C.B.; Franco, R.J.D.S. Aquatic exercise is as effective as dry land training to blood pressure reduction in postmenopausal hypertensive women. Physiother. Res. Int. 2014, 19, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Novais, I.P.; Jarrete, A.P.; Puga, G.M.; Araujo, H.N.; Delbin, M.A.; Zanesco, A. Effect of aerobic exercise training on cGMP levels and blood pressure in treated hypertensive postmenopausal women. Mot. Rev. Educação Física 2017, 23, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Shimojo, G.L.; Palma, R.K.; Brito, J.O.; Sanches, I.C.; Irigoyen, M.C.; De Angelis, K. Dynamic resistance training decreases sympathetic tone in hypertensive ovariectomized rats. Braz. J. Med. Biol. Res. 2015, 48, 523–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Palma, R.K.; Moraes-Silva, I.C.; da Silva Dias, D.; Shimojo, G.L.; Conti, F.F.; Bernardes, N.; Barboza, C.A.; Sanches, I.C.; da Rosa Araujo, A.S.; Irigoyen, M.C.; et al. Resistance or aerobic training decreases blood pressure and improves cardiovascular autonomic control and oxidative stress in hypertensive menopausal rats. J. Appl. Physiol. 2016, 121, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Auro, K.; Joensuu, A.; Fischer, K.; Kettunen, J.; Salo, P.; Mattsson, H.; Niironen, M.; Kaprio, J.; Eriksson, J.G.; Lehtimäki, T.; et al. A metabolic view on menopause and ageing. Nat. Commun. 2014, 5, 4708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaros, P.R.; Pires, C.E.; Bacci, M.; Moraes, C.; Zanesco, A. Effect of 6-months of physical exercise on the nitrate/nitrite levels in hypertensive postmenopausal women. BMC Womens Health 2009, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, T. Effects of aerobic exercise on blood pressure and lipids in overweight hypertensive postmenopausal women. J. Exerc. Rehabil. 2015, 11, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, T.L.; Lin, Y.Y.; Su, C.T.; Hu, C.C.; Yang, A.L. Improvement of acetylcholine-induced vasodilation by acute exercise in ovariectomized hypertensive rats. Chin. J. Physiol. 2016, 59, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Gielen, S.; Schuler, G.; Adams, V. Cardiovascular effects of exercise training: Molecular mechanisms. Circulation 2010, 122, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Maiorana, A.; O’Driscoll, G.; Taylor, R.; Green, D. Exercise and the nitric oxide vasodilator system. Sports Med. 2003, 33, 1013–1035. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, S.C.; Thijssen, D.H.; Green, D.J. Effects of exercise on endothelium and endothelium/smooth muscle cross talk: role of exercise-induced hemodynamics. J. Appl. Physiol. 2011, 111, 311–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardo, B.C.; Weeks, K.L.; Pretorius, L.; McMullen, J.R. Molecular distinction between physiological and pathological cardiac hypertrophy: Experimental findings and therapeutic strategies. Pharmacol. Ther. 2010, 128, 191–227. [Google Scholar] [CrossRef] [PubMed]
- Voloshenyuk, T.G.; Gardner, J.D. Estrogen improves TIMP-MMP balance and collagen distribution in volume-overloaded hearts of ovariectomized females. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R683–R693. [Google Scholar] [CrossRef] [PubMed]
- Diez, J. Mechanisms of cardiac fibrosis in hypertension. J. Clin. Hypertens. 2007, 9, 546–550. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Cheng, Y.J.; Hu, J.; Chu, L.X.; Shyu, W.C.; Kao, C.L.; Lin, T.B.; Kuo, C.H.; Yang, A.L.; Lee, S.D. The Coexistence of hypertension and ovariectomy additively increases cardiac apoptosis. Int. J. Mol. Sci. 2016, 17, 2036. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.A.; Claudio, E.R.; Mengal, V.; Oliveira, S.G.; Merlo, E.; Podratz, P.L.; Gouvea, S.A.; Graceli, J.B.; de Abreu, G.R. Exercise training reduces cardiac dysfunction and remodeling in ovariectomized rats submitted to myocardial infarction. PLoS ONE 2014, 9, e115970. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Yang, A.L.; Lin, Y.M.; Wu, F.N.; Lin, J.A.; Chan, Y.S.; Tsai, F.J.; Tsai, C.H.; Kuo, C.H.; Lee, S.D. Anti-apoptotic and pro-survival effects of exercise training on hypertensive hearts. J. Appl Physiol. 2012, 112, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.Y.; Lin, Y.Y.; Hsu, C.C.; Cheng, S.M.; Shyu, W.C.; Ting, H.; Yang, A.L.; Ho, T.J.; Lee, S.D. Antiapoptotic effect of exercise training on ovariectomized rat hearts. J. Appl. Physiol. 2016, 121, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; MacLaughlin, E.J.; et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [PubMed]


| Study | Age | Initial SBP/DBP | Number | Training Type | Training Intensity | Training Frequency | Outcomes |
|---|---|---|---|---|---|---|---|
| Staffileno et al., 2001 [27] | 57 ± 9 years | 150/94 mm Hg | pre: 9 post: 9 | Walk | Moderate (50–60% HRR) | 10min/time, 3 times/day, 5 days/week, 8 weeks |
|
| Latosik et al., 2014 [26] | N/A | 146/85 mm Hg | pre: 15 post: 15 | Walk | N/A | 8 weeks |
|
| Ammar 2015 [34] | 52–53 years | 152/94 mm Hg | pre: 15 post: 15 | Walktreadmill | Moderate (60–75% MHR) | 3 months |
|
| Zaros et al., 2009 [33] | 50 ± 4 years | 141/90 mm Hg | pre: 11 post: 11 | Cycle ergometer | Moderate (50% HRR) | 60 min/session, 3 times/week, for 6 months |
|
| Khalid et al., 2013 [21] | 53 ± 3 years | 148/94 mm Hg | pre: 18 post: 18 | Walktreadmill | Moderate (60–70% MHR) | Least 20 min/session, 3 times/week, for 8 weeks |
|
| Jarrete et al., 2014 [22] | 58 ± 1 years | 117/73 mm Hg | pre: 28 post: 28 | Walk | MLSS | 30–40 min/session, 3 times/week, for 8 weeks |
|
| Novais et al., 2017 [29] | 57 ± 1 years | 117/73 mm Hg | pre: 28 post: 28 | Walk | MLSS | 30–40 min/session, 3 times/week, for 8 weeks |
|
| Arca et al., 2014 [28] | 64 ± 7 years | 136/85 mm Hg | pre: 19 post: 19 | Aquaticwalk | Moderate (50–60% HRR) | 50 min/session, 3 times/week, for 12 weeks |
|
| Son et al., 2017 [23] | 75 ± 2 years | 145/95 mm Hg | pre: 10 post: 10 | Combined resistance and aerobic training | Moderate (40–70% HRR) | 70min/day, 3 times/week, for 12 weeks |
|
| Study | Age | Number | Training Type | Training Intensity | Training Frequency | Results |
|---|---|---|---|---|---|---|
| Marques et al., 2006 [16] | 14 weeks | Sed-int: 7 Sed-ovx:7 Ex-int: 7 Ex-ovx: 7 | Treadmill | Low | 60 min/day, 5 days/week, for 13 weeks |
|
| Sanches et al., 2012 [15] | 14 weeks | SHO:7 THO: 7 | Treadmill | Low- moderate(~50–60% maximal running speed) | 60 min/day, 5 days/week, for 8 weeks |
|
| Da Palma et al., 2016 [31] | 13 weeks | HS: 8 HSO: 8 HATO: 8 HRTO: 8 | Treadmill | Low-moderate(~50–60% maximal running speed) | 60 min /day, 5 days/week, for 8 weeks |
|
| Da Palma et al., 2016 [31] | 13 weeks | HS: 8 HSO: 8 HATO: 8 HRTO: 8 | Resistance | Moderate | 5 days/week, 8 weeks |
|
| Shimojo et al., 2015 [30] | 13 weeks | SC: 8 SH: 8 SHO: 8 RTHO: 8 | Resistance | Moderate | 5 days/week, 8 weeks |
|
| Claudio et al., 2017 [14] | 13 weeks | SH: 12 SSW:13 OVX: 13 OSW: 13 | Swimming | N/A | 60 min/day, 5 days/week, for 8 weeks |
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-Y.; Lee, S.-D. Cardiovascular Benefits of Exercise Training in Postmenopausal Hypertension. Int. J. Mol. Sci. 2018, 19, 2523. https://doi.org/10.3390/ijms19092523
Lin Y-Y, Lee S-D. Cardiovascular Benefits of Exercise Training in Postmenopausal Hypertension. International Journal of Molecular Sciences. 2018; 19(9):2523. https://doi.org/10.3390/ijms19092523
Chicago/Turabian StyleLin, Yi-Yuan, and Shin-Da Lee. 2018. "Cardiovascular Benefits of Exercise Training in Postmenopausal Hypertension" International Journal of Molecular Sciences 19, no. 9: 2523. https://doi.org/10.3390/ijms19092523