The Role of Potassium Channels in Arabidopsis thaliana Long Distance Electrical Signalling: AKT2 Modulates Tissue Excitability While GORK Shapes Action Potentials
Abstract
:1. Introduction
2. Results
2.1. Electric Stimulation Induces Action Potentials in Arabidopsis
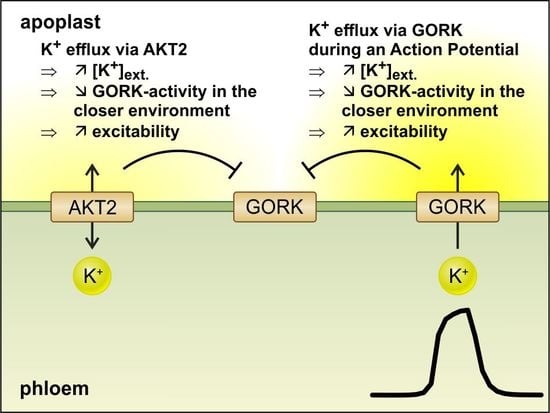
2.2. The Outward-Rectifying Channel GORK Limits Membrane Depolarisation and Accelerates Its Repolarisation
2.3. The Weakly-Rectifying Shaker-Like Channel AKT2 Affects Plant Excitability by Virtue of Its Rectification Mode
3. Discussion
3.1. Plant APs Could Be Carried by Anions and K+
3.2. Computational Simulation of Plant AP Generation and Propagation Allows Mechanistic Insight into the Contribution of GORK
3.3. Mechanism of Plant AP Propagation—Likely Not a Pure Electric Phenomenon
3.4. Integration into Plant Physiology
4. Materials and Methods
4.1. Plant Material
4.2. Arabidopsis Leaf with Stimulation and Recording Electrodes
4.3. Modelling Approach
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Computational Simulation of Action Potentials Using the Cable Equation
| Algorithms |
|
References
- Sukhov, V.; Gaspirovich, V.; Mysyagin, S.; Vodeneev, V. High-temperature tolerance of photosynthesis can be linked to local electrical responses in leaves of pea. Front. Physiol. 2017, 8, 763. [Google Scholar] [CrossRef] [PubMed]
- Davies, E. New functions for electrical signals in plants. New Phytol. 2004, 161, 607–610. [Google Scholar] [CrossRef]
- Sukhov, V. Electrical signals as mechanism of photosynthesis regulation in plants. Photosynth. Res. 2016, 130, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Sukhova, E.; Akinchits, E.; Sukhov, V. Mathematical models of electrical activity in plants. J. Membr. Biol. 2017, 250, 407–423. [Google Scholar] [CrossRef] [PubMed]
- Fromm, J.; Lautner, S. Electrical signals and their physiological significance in plants. Plant Cell Environ. 2007, 30, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Trębacz, K.; Dziubińska, H.; Król, E. Electrical signals in long-distance communication in plants. In Communication in Plants. Neuronal Aspects of Plant Life; Baliska, F., Mancuso, S., Volkmann, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 277–290. [Google Scholar]
- Choi, W.G.; Hilleary, R.; Swanson, S.J.; Kim, S.H.; Gilroy, S. Rapid, long-distance electrical and calcium signaling in plants. Annu. Rev. Plant Biol. 2016, 67, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Homann, U.; Thiel, G. Cl− and K+ channel currents during the action potential in Chara. Simultaneous recording of membrane voltage and patch currents. J. Membr. Biol. 1994, 141, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Beilby, M.J. Action potential in charophytes. Int. Rev. Cytol. 2007, 257, 43–82. [Google Scholar] [PubMed]
- Beilby, M.J.; Walker, N.A. Modeling the current-voltage characteristics of Chara membranes: I. The effect of ATP removal and zero turgor. J. Membr. Biol. 1996, 149, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Mueller-Roeber, B.; Koehler, B. Voltage-gated ion channels. In Membrane Transport in Plants; Blatt, M.R., Ed.; Blackwell Publishing: Oxford, UK, 2004; pp. 150–192. [Google Scholar]
- Vodeneev, V.A.; Opritov, V.A.; Pyatygin, S.S. Reversible changes of extracellular pH during action potential generation in a higher plant Cucurbita pepo. Russ. J. Plant Physiol. 2006, 53, 481–487. [Google Scholar] [CrossRef]
- Sukhov, V.; Vodeneev, V. A mathematical model of action potential in cells of vascular plants. J. Membr. Biol. 2009, 232, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Sukhov, V.; Nerush, V.; Orlova, L.; Vodeneev, V. Simulation of action potential propagation in plants. J. Theor. Biol. 2011, 291, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Hills, A.; Batz, U.; Amtmann, A.; Lew, V.L.; Blatt, M.R. Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol. 2012, 159, 1235–1251. [Google Scholar] [CrossRef] [PubMed]
- Minguet-Parramona, C.; Wang, Y.; Hills, A.; Vialet-Chabrand, S.; Griffiths, H.; Rogers, S.; Lawson, T.; Lew, V.L.; Blatt, M.R. An optimal frequency in Ca2+ oscillations for stomatal closure is an emergent property of ion transport in guard cells. Plant Physiol. 2016, 170, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Davies, E. Electrical signals in plants: Facts and hypothesis. In Plant Electrophysiology, Theory and Methods, Volkov, A.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 407–422. [Google Scholar]
- Felle, H.H.; Zimmermann, M.R. Systemic signalling in barley through action potentials. Planta 2007, 226, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Chauvin, A.; Pascaud, F.; Kellenberger, S.; Farmer, E.E. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 2013, 500, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Stephens, N.R.; Qi, Z.; Spalding, E.P. Glutamate receptor subtypes evidenced by differences in desensitization and dependence on the GLR3.3 and GLR3.4 genes. Plant Physiol. 2008, 146, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Tapken, D.; Anschutz, U.; Liu, L.H.; Huelsken, T.; Seebohm, G.; Becker, D.; Hollmann, M. A plant homolog of animal glutamate receptors is an ion channel gated by multiple hydrophobic amino acids. Sci. Signal. 2013, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Ramirez, C.; Michard, E.; Simon, A.A.; Damineli, D.S.C.; Hernandez-Coronado, M.; Becker, J.D.; Feijo, J.A. GLUTAMATE RECEPTOR-LIKE channels are essential for chemotaxis and reproduction in mosses. Nature 2017, 549, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Michard, E.; Lima, P.T.; Borges, F.; Silva, A.C.; Portes, M.T.; Carvalho, J.E.; Gilliham, M.; Liu, L.H.; Obermeyer, G.; Feijo, J.A. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 2011, 332, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Gomez-Porras, J.L.; Riano-Pachon, D.M.; Hedrich, R.; Geiger, D. Molecular evolution of slow and quick anion channels (SLACs and QUACs/ALMTs). Front. Plant Sci. 2012, 3, 263. [Google Scholar] [CrossRef] [PubMed]
- Imes, D.; Mumm, P.; Bohm, J.; Al-Rasheid, K.A.; Marten, I.; Geiger, D.; Hedrich, R. Open stomata 1 (OST1) kinase controls R-type anion channel QUAC1 in Arabidopsis guard cells. Plant J. 2013, 74, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, R.; Geiger, D. Biology of SLAC1-type anion channels—from nutrient uptake to stomatal closure. New Phytol. 2017, 216, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Duby, G.; Boutry, M. The plant plasma membrane proton pump ATPase: A highly regulated P-type ATPase with multiple physiological roles. Pflugers Arch. 2009, 457, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, R.; Salvador-Recatala, V.; Dreyer, I. Electrical wiring and long-distance plant communication. Trends Plant Sci. 2016, 21, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, B.; Pilot, G.; Michard, E.; Gaymard, F.; Sentenac, H.; Thibaud, J.B. A shaker-like K+ channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell 2000, 12, 837–851. [Google Scholar] [PubMed]
- Marten, I.; Hoth, S.; Deeken, R.; Ache, P.; Ketchum, K.A.; Hoshi, T.; Hedrich, R. AKT3, a phloem-localized K+ channel, is blocked by protons. Proc. Natl. Acad. Sci. USA 1999, 96, 7581–7586. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Blatt, M.R. What makes a gate? The ins and outs of Kv-like K+ channels in plants. Trends Plant Sci. 2009, 14, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Hoth, S.; Ache, P.; Wenkel, S.; Roelfsema, M.R.; Meyerhoff, O.; Hartung, W.; Hedrich, R. Regulation of the ABA-sensitive Arabidopsis potassium channel gene GORK in response to water stress. FEBS Lett. 2003, 554, 119–126. [Google Scholar] [CrossRef]
- Favre, P.; Agosti, R.D. Voltage-dependent action potentials in Arabidopsis thaliana. Physiol. Plant. 2007, 131, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, B.; Davies, E. Both action potentials and variation potentials induce proteinase inhibitor gene expression in tomato. FEBS Lett. 1996, 390, 275–279. [Google Scholar] [CrossRef]
- Herde, O.; Fuss, H.; Peña-Cortés, H.; Fisahn, J. Proteinase inhibitor II gene expression induced by electrical stimulation and control of photosynthetic activity in tomato plants. Plant Cell Physiol. 1995, 36, 737–742. [Google Scholar]
- Fromm, J.; Bauer, T. APs in maize sieve tubes change phloem translocation. J. Exp. Bot. 1994, 45, 463–469. [Google Scholar] [CrossRef]
- Herde, O.; Atzorn, R.; Fisahn, J.; Wasternack, C.; Willmitzer, L.; Pena Cortes, H. Localized wounding by heat initiates the accumulation of proteinase inhibitor ii in abscisic acid-deficient plants by triggering jasmonic acid biosynthesis. Plant Physiol. 1996, 112, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Bean, B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007, 8, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Michard, E.; Dreyer, I.; Lacombe, B.; Sentenac, H.; Thibaud, J.B. Inward rectification of the AKT2 channel abolished by voltage-dependent phosphorylation. Plant J. 2005, 44, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Michard, E.; Lacombe, B.; Thibaud, J.B. A plant Shaker-like K+ channel switches between two distinct gating modes resulting in either inward-rectifying or “leak” current. FEBS Lett. 2001, 505, 233–239. [Google Scholar] [CrossRef]
- Gajdanowicz, P.; Michard, E.; Sandmann, M.; Rocha, M.; Correa, L.G.; Ramirez-Aguilar, S.J.; Gomez-Porras, J.L.; Gonzalez, W.; Thibaud, J.B.; van Dongen, J.T.; et al. Potassium (K+) gradients serve as a mobile energy source in plant vascular tissues. Proc. Natl. Acad. Sci. USA 2011, 108, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Michard, E.; Lacombe, B.; Poree, F.; Mueller-Roeber, B.; Sentenac, H.; Thibaud, J.B.; Dreyer, I. A unique voltage sensor sensitizes the potassium channel AKT2 to phosphoregulation. J. Gen. Physiol. 2005, 126, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, M.; Sklodowski, K.; Gajdanowicz, P.; Michard, E.; Rocha, M.; Gomez-Porras, J.L.; Gonzalez, W.; Correa, L.G.; Ramirez-Aguilar, S.J.; Cuin, T.A.; van Dongen, J.T.; Thibaud, J.B.; Dreyer, I. The K+ battery-regulating Arabidopsis K+ channel AKT2 is under the control of multiple post-translational steps. Plant Signal. Behav. 2011, 6, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Hodick, D.; Sievers, A. On the mechanism of trap closure of Venus flytrap (Dionaea muscipula Ellis). Planta 1989, 179, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Hosy, E.; Vavasseur, A.; Mouline, K.; Dreyer, I.; Gaymard, F.; Poree, F.; Boucherez, J.; Lebaudy, A.; Bouchez, D.; Very, A.A.; et al. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA 2003, 100, 5549–5554. [Google Scholar] [CrossRef] [PubMed]
- Gold, C.; Henze, D.A.; Koch, C. Using extracellular action potential recordings to constrain compartmental models. J. Comput. Neurosci. 2007, 23, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Pods, J. A comparison of computational models for the extracellular potential of neurons. J. Integr. Neurosci. 2017, 16, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Gomez-Porras, J.L.; Riedelsberger, J. The potassium battery: A mobile energy source for transport processes in plant vascular tissues. New Phytol. 2017, 216, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Wulfetange, K.; Poree, F.; Michard, E.; Gajdanowicz, P.; Lacombe, B.; Sentenac, H.; Thibaud, J.B.; Mueller-Roeber, B.; Blatt, M.R.; et al. External K+ modulates the activity of the Arabidopsis potassium channel SKOR via an unusual mechanism. Plant J. 2006, 46, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vallet, A.; Lopez, G.; Ramos, B.; Delgado-Cerezo, M.; Riviere, M.P.; Llorente, F.; Fernandez, P.V.; Miedes, E.; Estevez, J.M.; Grant, M.; et al. Disruption of abscisic acid signaling constitutively activates Arabidopsis resistance to the necrotrophic fungus Plectosphaerella cucumerina. Plant Physiol. 2012, 160, 2109–2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lackman, P.; Gonzalez-Guzman, M.; Tilleman, S.; Carqueijeiro, I.; Perez, A.C.; Moses, T.; Seo, M.; Kanno, Y.; Hakkinen, S.T.; Van Montagu, M.C.; et al. Jasmonate signaling involves the abscisic acid receptor PYL4 to regulate metabolic reprogramming in Arabidopsis and tobacco. Proc. Natl. Acad. Sci. USA 2011, 108, 5891–5896. [Google Scholar] [CrossRef] [PubMed]
- Cherel, I.; Michard, E.; Platet, N.; Mouline, K.; Alcon, C.; Sentenac, H.; Thibaud, J.B. Physical and functional interaction of the Arabidopsis K+ channel AKT2 and phosphatase AtPP2CA. Plant Cell 2002, 14, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Herde, O.; Pena Cortes, H.; Wasternack, C.; Willmitzer, L.; Fisahn, J. Electric signaling and Pin2 gene expression on different abiotic stimuli depend on a distinct threshold level of endogenous abscisic acid in several abscisic acid-deficient tomato mutants. Plant Physiol. 1999, 119, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Choi, W.G.; Gilroy, S.; Morris, R.J. A ROS-assisted calcium wave dependent on the AtRBOHD NADPH oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol. 2016, 171, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Vincent, T.R.; Avramova, M.; Canham, J.; Higgins, P.; Bilkey, N.; Mugford, S.T.; Pitino, M.; Toyota, M.; Gilroy, S.; Miller, A.J.; et al. Interplay of plasma membrane and vacuolar ion channels, together with BAK1, elicits rapid cytosolic calcium elevations in Arabidopsis during aphid feeding. Plant Cell 2017, 29, 1460–1479. [Google Scholar] [CrossRef] [PubMed]
- Sklodowski, K.; Riedelsberger, J.; Raddatz, N.; Riadi, G.; Caballero, J.; Cherel, I.; Schulze, W.; Graf, A.; Dreyer, I. The receptor-like pseudokinase MRH1 interacts with the voltage-gated potassium channel AKT2. Sci. Rep. 2017, 7, 44611. [Google Scholar] [CrossRef] [PubMed]
- Dennison, K.L.; Robertson, W.R.; Lewis, B.D.; Hirsch, R.E.; Sussman, M.R.; Spalding, E.P. Functions of AKT1 and AKT2 potassium channels determined by studies of single and double mutants of Arabidopsis. Plant Physiol. 2001, 127, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Ache, P.; Becker, D.; Ivashikina, N.; Dietrich, P.; Roelfsema, M.R.; Hedrich, R. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett. 2000, 486, 93–98. [Google Scholar] [CrossRef]
- Schott, S.; Valdebenito, B.; Bustos, D.; Gomez-Porras, J.L.; Sharma, T.; Dreyer, I. Cooperation through competition-dynamics and microeconomics of a minimal nutrient trade system in arbuscular mycorrhizal symbiosis. Front. Plant Sci. 2016, 7, 912. [Google Scholar] [CrossRef] [PubMed]




| WS Ecotype | Col-0 Ecotype | |||
|---|---|---|---|---|
| WT | gork | WT | gork | |
| Excitability (%) | 51% | 59% | 88% | 73% |
| Amplitude, E1 (mV) | 44.6 ± 10.4 | 50.4 ± 13.6 | 58.3 ± 5.9 | 63.8 ± 21.8 |
| Amplitude, E2 (mV) | 47.2 ± 11.2 | 81.8 ± 8.9 | 62.2 ± 5.5 | 93.6 ± 13.3 |
| Width, E1 (s) | 6.7 ± 1.7 | 13.6 ± 3.4 | 6.6 ± 2.4 | 13.6 ± 6.4 |
| Width, E2 (s) | 6.7 ± 1.6 | 18.1 ± 5.0 | 7.6 ± 1.5 | 19.7 ± 6.9 |
| Speed (mm s−1) | 1.3 ± 0.3 | 1.1 ± 0.6 | 1.2 ± 0.4 | 1.2 ± 0.3 |
| Line | WS Ecotype | Col-0 Ecotype | |||||
|---|---|---|---|---|---|---|---|
| AKT2-KS | AKT2-AA | akt2-1 | WT | AKT2-NN | akt2-2 | WT | |
| Excitability (%) | 26% | 31% | 41% | 51% | 59% | 63% | 88% |
| Amplitude (mV) | 43.2 ± 14.5 | 41.0 ± 7.3 | 38.1 ± 10.6 | 47.2 ± 11.2 | 53.0 ± 9.2 | 52.0 ± 12.3 | 62.2 ± 5.5 |
| Width (s) | 7.8 ± 2.8 | 7.7 ± 1.9 | 6.6 ± 2.1 | 10.9 ± 4.2 | 6.7 ± 1.6 | 7.8 ± 2.5 | 7.8 ± 1.5 |
| Speed (mm/s) | 0.9 ± 0.3 | 1.3 ± 0.4 | 1.2 ± 0.4 | 1.3 ± 0.3 | 1.1 ± 0.4 | 1.0 ± 0.2 | 1.2 ± 0.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuin, T.A.; Dreyer, I.; Michard, E. The Role of Potassium Channels in Arabidopsis thaliana Long Distance Electrical Signalling: AKT2 Modulates Tissue Excitability While GORK Shapes Action Potentials. Int. J. Mol. Sci. 2018, 19, 926. https://doi.org/10.3390/ijms19040926
Cuin TA, Dreyer I, Michard E. The Role of Potassium Channels in Arabidopsis thaliana Long Distance Electrical Signalling: AKT2 Modulates Tissue Excitability While GORK Shapes Action Potentials. International Journal of Molecular Sciences. 2018; 19(4):926. https://doi.org/10.3390/ijms19040926
Chicago/Turabian StyleCuin, Tracey Ann, Ingo Dreyer, and Erwan Michard. 2018. "The Role of Potassium Channels in Arabidopsis thaliana Long Distance Electrical Signalling: AKT2 Modulates Tissue Excitability While GORK Shapes Action Potentials" International Journal of Molecular Sciences 19, no. 4: 926. https://doi.org/10.3390/ijms19040926