Pereskia aculeata Muller (Cactaceae) Leaves: Chemical Composition and Biological Activities
Abstract
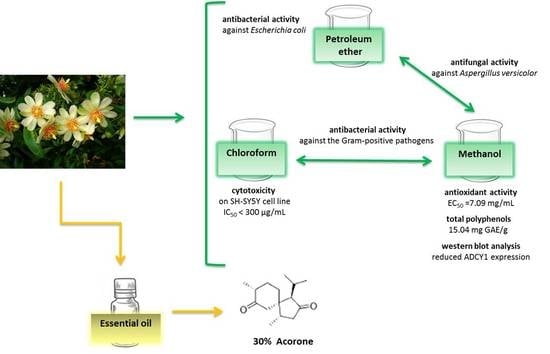
:1. Introduction
2. Results
2.1. Essential Oil Yield and Composition
2.2. Free Radical-Scavenging Capacity
2.3. Total Phenolic Compounds
2.4. Antimicrobial and Antifungal Activity
2.5. Cytotoxicity of Pereskia aculeata Extracts
2.6. Adenylate Cyclase 1 (ADCY1): Western Blot Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction Procedure
4.3. Isolation of the Volatile Oil
4.4. GC-FID Analysis
4.5. GC/MS Analysis
4.6. Identification of the Essential Oil Components
4.7. Free Radical-Scavenging Capacity
4.7.1. Sample Preparations
4.7.2. DPPH Radical
4.8. Total Phenolic Compounds
4.9. Antimicrobial Assays
4.10. Antifungal Activity
4.11. Cell Cultures
4.12. MTT Assay
4.13. Extraction Proteins and Western Blotting
4.14. Statistical Analysis
Author Contributions
Conflicts of Interest
References
- Sharif, K.M.; Rahman, M.M.; Zaidul, I.S.M.; Jannatul, A.; Akanda, M.J.H.; Mohamed, A.; Shamsudin, S.H. Pharmacological relevance of primitive leafy Cactuses Pereskia. Res. J. Biotechnol. 2013, 8, 134–142. [Google Scholar]
- Pinto, N.D.C.C.; Scio, E. The biological activities and chemical composition of Pereskia species (Cactaceae)—A review. Plant Food Hum. Nutr. 2014, 69, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.E.F.; Corrêa, A.D. Utilization of cacti of the genus Pereskia in the human diet in a municipality of Minas Gerais. Ciência Rural 2012, 42, 751–756. [Google Scholar] [CrossRef]
- Martinevski, C.S.; Oliveira, V.R.; Rios, A.D.O.; Flores, S.H.; Venzke, J.G. Utilization of Bertalha (Anredera cordifolia (Ten.) Steenis) and ora pro nobis (Pereskia aculeata Mill.) in preparing breads. Braz. J. Food Nutr. 2013, 24, 272. [Google Scholar]
- Takeiti, C.Y.; Antonio, G.C.; Motta, E.M.; Collares-Queiroz, F.P.; Park, K.J. Nutritive evaluation of non-conventional leafy vegetable (Pereskia aculeata Miller). Int. J. Food Sci. Nutr. 2009, 60, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.C.C.; Santos, R.C.; Machado, D.C.; Florêncio, J.R.; Fagundes, E.M.Z.; Antinarelli, L.M.R.; Scio, E. Cytotoxic and antioxidant activity of Pereskia aculeata Miller. Pharmacologyonline 2012, 3, 63–69. [Google Scholar]
- Sim, K.S.; Sri Nurestri, A.M.; Sinniah, S.K.; Kim, K.H.; Norhanom, A.W. Acute oral toxicity of Pereskia bleo and Pereskia grandifolia in mice. Pharmacogn. Mag. 2010, 6, 67–70. [Google Scholar] [PubMed]
- Malek, S.N.A.; Shin, S.K.; Wahab, N.A.; Yaacob, H. Cytotoxic components of Pereskia bleo (Kunth) DC. (Cactaceae) leaves. Molecules 2009, 14, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.F.; de Barros, I.B.; Mancini, E.; de Martino, L.; Scandolera, E.; de Feo, V. Chemical composition and biological activities of the essential oils from two Pereskia species grown in Brazil. Nat. Prod. Commun. 2014, 9, 1805–1808. [Google Scholar] [PubMed]
- Misharina, T.A. Influence of the duration and conditions of storage on the composition of the essential oil from coriander seeds. Appl. Biochem. Microbiol. 2001, 37, 622–628. [Google Scholar] [CrossRef]
- Wahab, S.I.A.; Abdul, A.B.; Mohan, S.M.; Al-Zubairi, A.S.; Elhassan, M.M.; Ibrahim, M.Y. Biological activities of Pereskia bleo extracts. Int. J. Pharmacol. 2009, 5, 71–75. [Google Scholar] [CrossRef]
- Sim, K.S.; Sri Nurestri, A.M.; Norhanom, A.W. Phenolic content and antioxidant activity of Pereskia grandifolia Haw. (Cactaceae) extracts. Pharmacogn. Mag. 2010, 6, 248–254. [Google Scholar] [PubMed]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2009, 113, 1226–33. [Google Scholar] [CrossRef]
- Del Monte, D.; de Martino, L.; Marandino, A.; Fratianni, F.; Nazzaro, F.; de Feo, V. Phenolic content, antimicrobial and antioxidant activities of Hypericum perfoliatum L. Ind. Crops Prod. 2015, 74, 342–347. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; de Martino, L.; Coppola, R.; de Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Riccardi, R.; Spigno, P.; Ombra, M.N.; Cozzolino, A.; Tremonte, P.; Nazzaro, F. Biochemical characterization and antimicrobial and antifungal activity of two endemic varieties of garlic (Allium sativum L.) of the campania region, southern Italy. J. Med. Food 2016, 19, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Çobankara, F.K.; Altinöz, H.C.; Erganiş, O.; Kav, K.; Belli, S. In vitro antibacterial activities of root-canal sealers by using two different methods. J. Endod. 2004, 30, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, S.; Domig, K.J.; Mair, C.; Zitz, U.; Huys, G.; Kneifel, W. Comparison of broth microdilution, Etest, and agar disk diffusion methods for antimicrobial susceptibility testing of Lactobacillus acidophilus group members. Appl. Environ. Microbiol. 2008, 74, 3745–3748. [Google Scholar] [CrossRef] [PubMed]
- Saritha, K.; Rajesh, A.; Manjulatha, K.; Setty, O.H.; Yenugu, S. Mechanism of antibacterial action of the alcoholic extracts of Hemidesmus indicus (L.) R. Br. ex Schult, Leucas aspera (Wild.), Plumbago zeylanica L., and Tridax procumbens (L.) R. Br. ex Schult. Front. Microbiol. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Philip, K.; Malek, S.N.A.; Sani, W.; Shin, S.K.; Kumar, S.; Lai, H.S.; Serm, L.G.; Rahman, S.N.S.A. Antimicrobial activity of some medicinal plants from Malaysia. Am. J. Appl. Sci. 2009, 6, 1613–1617. [Google Scholar]
- Salt, T.A.; Tocker, J.E.; Adler, J.H. Dominance of Δ5-sterols in eight species of the Cactaceae. Phytochemistry 1987, 26, 731–733. [Google Scholar] [CrossRef]
- Ling, W.H.; Jones, P.J.H. Minireview dietary phytosterols: A review of metabolism, benefits and side effects. Life Sci. 1995, 57, 195–206. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; Macdonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumours and other biological systems. Cancer Chemother. Rep. 1972, 3, 59–61. [Google Scholar]
- Tan, M.L.; Sulaiman, S.F.; Najimuddin, N.; Samian, M.R.; Muhammad, T.T. Methanolic extract of Pereskia bleo (Kunth) DC. (Cactaceae) induces apoptosis in breast carcinoma, T47-D cell line. J. Ethnopharmacol. 2005, 96, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.Y.; Stanbridge, E.J.; Yusoff, K.; Shafee, N. Hypoxia affects cellular responses to plant extracts. J. Ethnopharmacol. 2012, 144, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, F.C.F.; Correia, N.D.A.; Albuquerque, K.L.G.D.; De Sousa, D.P.; Da Rosa, M.R.D.; Pimenta, M.B.F.; de Almeida, R.N. Naturally occurring anxiolytic substances from aromatic plants of genus Citrus. J. Med. Plant Res. 2012, 6, 342–347. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, D.H.; Park, S.J.; Park, D.H.; Jung, S.Y.; Kim, H.J.; Lee, Y.S.; Jin, C.; Ryu, J.H. The n-butanolic extract of Opuntia ficus-indica var. saboten enhances long-term memory in the passive avoidance task in mice. Biol. Psychiatry 2010, 2, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Wahab, I.R.; Guilhon, C.C.; Fernandes, P.D.; Boylan, F. Anti-nociceptive activity of Pereskia bleo Kunth. (Cactaceae) leaves extracts. J. Ethnopharmacol. 2012, 144, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Guilhon, C.C.; Abdul Wahab, I.R.; Boylan, F.; Fernandes, P.D. Central antinociceptive and mechanism of action of Pereskia bleo Kunth. leaves crude extract, fractions, and isolated compounds. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Elisabetsky, E.; Silva Brum, L.F.; Souza, D.O. Anticonvulsant properties of linalool in glutamate-related seizure models. Phytomedicine 1999, 6, 107–113. [Google Scholar] [CrossRef]
- Davis, M.I.; Ronesi, J.; Lovinger, D.M. A predominant role for inhibition of the adenylate cyclase/protein kinase A pathway in ERK activation by cannabinoid receptor 1 in N1E-115 neuroblastoma cells. J. Biol. Chem. 2003, 278, 48973–48980. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.S.; Hocker, H.J; Gorfe, A.A.; Cavasotto, C.N.; Dessauer, C.W. Isoform selectivity of adenylyl cyclase inhibitors: Characterization of known and novel compounds. J. Pharmacol. Exp. Ther. 2013, 347, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Pharmacopeia, 5th ed.; Council of Europe: Strasbourg Cedex, France, 2004; Volume I, pp. 217–218. [Google Scholar]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavour and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicone and Carbowax 20M phases. J. Chromatogr. 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Goodner, K.L. Practical retention index models of OV-101, DB-1, DB-5, and DB-Wax for flavor and fragrance compounds. LWT-Food Sci. Technol. 2008, 41, 951–958. [Google Scholar] [CrossRef]
- John Wiley & Sons. Wiley Registry of Mass Spectral Data, with NIST Spectral Data CD Rom, 7th ed.; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Picerno, P.; Autore, G.; Marzocco, S.; Meloni, M.; Sanogo, R.; Aquino, R.P. Anti-inflammatory activity of verminoside from Kigelia africana and evaluation of cutaneous irritation in cell cultures and reconstituted human epidermis. J. Nat. Prod. 2005, 68, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Petrella, A.; Ercolino, S.F.; Festa, M.; Gentilella, A.; Tosco, A.; Conzen, S.D.; Parente, L. Dexamethasone inhibits TRAIL-induced apoptosis of thyroid cancer cells via Bcl-XL induction. Eur. J. Cancer 2006, 42, 3287–3293. [Google Scholar]




| No. | Compound | % | Ki a | Ki b | Identification c |
|---|---|---|---|---|---|
| 1 | (E)-β-Ionone | 0.75 | 1488 | 1476 | 1,2 |
| 2 | Dihydro-β-agarofuran | 0.57 | 1519 | 1503 | 1,2 |
| 3 | cis-Dihydro-mayurone | 0.17 | 1551 | 1595 | 1,2 |
| 4 | Caryophyllene oxide | 0.51 | 1575 | 1583 | 1,2 |
| 5 | α-Muurolol | 0.22 | 1643 | 1642 | 1,2,3 |
| 6 | ar-Tumerone | 1.10 | 1654 | 1668 | 1,3 |
| 7 | 14-Hydroxy-(Z)-caryophyllene | 0.29 | 1661 | 1667 | 1,2 |
| 8 | (Z)-3-Hexenyl salicylate | 0.17 | 1664 | 1669 | 1,2 |
| 9 | 14-Hydroxy-9-epi-(E)-caryophyllene | 0.28 | 1668 | 1669 | 1,2 |
| 10 | 2-hexyl-(E)-cinnamaldehyde | 0.60 | 1734 | 1749 | 1,2 |
| 11 | 1-Octadecene | 0.62 | 1783 | 1790 | 1,2,3 |
| 12 | 2-Ethylhexyl salicylate | 1.73 | 1789 | 1807 | 1,2 |
| 13 | Acorone | 30.0 | 1824 | 1820 | 1,2 |
| 14 | Cyclopentadecanolide | 5.48 | 1830 | 1833 | 1,2 |
| 15 | 1-Nonadecen-ol | 6.18 | 1848 | 1,2 | |
| 16 | (Z,Z)-Methyl-4,6-hexadecadiene | 16.34 | 1866 | 1,2 | |
| 18 | (5E,9E)-Farnesyl acetone | 5.70 | 1903 | 1913 | 1,2 |
| 19 | Methyl hexadecanoate | 4.92 | 1910 | 1921 | 1,2,3 |
| 20 | Isopropyl hexadecanoate | 0.42 | 1984 | 2024 | 1,2 |
| 21 | Methyl linoleate | 4.44 | 2075 | 2085 | 1,2 |
| 22 | Methyl octadecanoate | 0.69 | 2107 | 2125 | 1,2,3 |
| 23 | Linoleic acid | 4.74 | 2126 | 2133 | 1,2 |
| 24 | Phytol | 5.11 | 2148 | 1,2 | |
| Total compounds | 91.03 | ||||
| Oxygenated monoterpenes | 15.96 | ||||
| Monoterpene hydrocarbons | 0.62 | ||||
| Oxygenated sesquiterpenes | 44.92 | ||||
| Non-terpene | 24.42 | ||||
| Diterpene hydrocarbons | 5.11 | ||||
| Sample | Equation | R2 | EC50 |
|---|---|---|---|
| Petroleum ether | y = 0.3072x − 6.1561 | 0.9811 | 18.27 mg/mL |
| Chloroform | y = 0.6346x − 1.0909 | 0.8999 | 81.09 mg/mL |
| Methanol | y = 7.8743x − 5.8489 | 0.9938 | 7.09 mg/mL |
| Ether Extract mg GAE/g | Chloroform Extract mg GAE/g | Methanol Extract mg GAE/g | |
|---|---|---|---|
| Total polyphenols | 11.78 ± 0.23 | 5.17 ± 0.41 | 15.04 ± 0.67 |
| Extract | B. cereus 4313 | B. cereus 4384 | S. aureus | P. aeruginosa | E. coli 8579 |
|---|---|---|---|---|---|
| Petroleum ether | |||||
| 1 µg/mL | 7.60 ± 0.47 *** | 3.66 ± 0.47 **** | 13.00 ± 1.41 **** | 4.0± 00 **** | 10.66 ± 0.47 **** |
| 2 µg/mL | 11.33 ± 0.94 | 5.66 ± 0.47 **** | 16.66 ± 1.24 **** | 7.33 ± 0.94 *** | 18.33 ± 1.59 **** |
| 4 µg/mL | 14.66 ± 0.47 **** | 10.0 ± 00 | 20.33 ± 00 **** | 10.66 ± 1.24 | 20.66 ± 0.94 **** |
| Chloroform | |||||
| 1 µg/mL | 2.00 ± 00 **** | 7.66 ± 0.94 **** | 5.66 ± 0.47 | 8.66 ± 0.47 *** | 0 |
| 2 µg/mL | 4.33 ± 0.47 **** | 9.33 ± 0.47 * | 8.33 ± 1.24 **** | 8.66 ± 0.47 *** | 0 |
| 4 µg/mL | 6.33 ± 0.47 **** | 9.33 ± 0.47 * | 10.33 ± 0.47 **** | 10.33 ± 0.47 | 0 |
| Methanol | |||||
| 1 µg/mL | 0 | 7.66 ± 0.47 **** | 0 | 6.33 ± 0.47 **** | 0 |
| 2 µg/mL | 5.33 ± 0.47 **** | 8.66 ± 0.47 ** | 7.6 ± 1.88 * | 10.66 ± 0.47 | 0 |
| 4 µg/mL | 10.66 ± 00 | 8.66 ± 0.47 ** | 8.33 ± 1.24 *** | 13.33 ± 1.24 **** | 0 |
| DMSO | |||||
| Control | 10.5 ± 0.5 | 10.5 ± 0.5 | 6.0 ± 0.5 | 10.5 ± 0.5 | 6.0 ± 0.5 |
| Extract | P. expansum | P. citrinum | A. niger | A. versicolor |
|---|---|---|---|---|
| Petroleum ether | ||||
| 1 µg/mL | – | – | – | 2.33 ± 0.47 |
| 2 µg/mL | – | – | – | 6.66 ± 0.47 |
| 4 µg/mL | 5.33 ± 0.47 | 5.33 ± 0.47 | 2.00 ± 00 | 9.33 ± 0.47 |
| Chloroform | ||||
| 1 µg/mL | – | – | – | – |
| 2 µg/mL | – | – | – | – |
| 4 µg/mL | – | 2.66 ± 0.47 | – | 5.00 ± 00 |
| Methanol | ||||
| 1 µg/mL | – | – | – | 2.33 ± 0.47 |
| 2 µg/mL | 2.70 ± 0.47 | – | – | 5.00 ± 00 |
| 4 µg/mL | 5.33 ± 0.47 | 4.66 ± 00 | – | 6.66 ± 00 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, L.F.; Caputo, L.; Inchausti De Barros, I.B.; Fratianni, F.; Nazzaro, F.; De Feo, V. Pereskia aculeata Muller (Cactaceae) Leaves: Chemical Composition and Biological Activities. Int. J. Mol. Sci. 2016, 17, 1478. https://doi.org/10.3390/ijms17091478
Souza LF, Caputo L, Inchausti De Barros IB, Fratianni F, Nazzaro F, De Feo V. Pereskia aculeata Muller (Cactaceae) Leaves: Chemical Composition and Biological Activities. International Journal of Molecular Sciences. 2016; 17(9):1478. https://doi.org/10.3390/ijms17091478
Chicago/Turabian StyleSouza, Lucèia Fàtima, Lucia Caputo, Ingrid Bergman Inchausti De Barros, Florinda Fratianni, Filomena Nazzaro, and Vincenzo De Feo. 2016. "Pereskia aculeata Muller (Cactaceae) Leaves: Chemical Composition and Biological Activities" International Journal of Molecular Sciences 17, no. 9: 1478. https://doi.org/10.3390/ijms17091478