Evaluation of Pulmonary Toxicity of Zinc Oxide Nanoparticles Following Inhalation and Intratracheal Instillation
Abstract
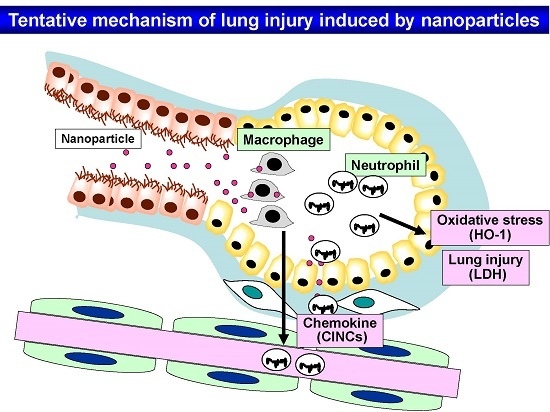
:1. Introduction
2. Results
2.1. Intratracheal Instillation Study
2.1.1. Cell Analysis in Bronchoalveolar Lavage Fluid (BALF)
2.1.2. Cytokine-Induced Neutrophil Chemoattractant (CINC) Concentration in BALF
2.1.3. Heme Oxigenase-1 (HO-1) Concentration in BALF
2.1.4. Histopathological Changes in the Lungs
2.1.5. Morphological Features of Alveolar Macrophages by TEM
3. Inhalation Study
3.1. Cell Analysis in BALF
3.2. CINC Concentration in BALF
3.3. HO-1 Concentration in BALF
3.4. Histopathological Changes in the Lungs
3.5. Morphological Features of Alveolar Macrophages by TEM
4. Discussion
5. Methods and Materials
5.1. Sample Preparation of ZnO Nanoparticle Suspensions
5.2. Animals
5.3. Intratracheal Instillation of ZnO Nanoparticles
5.4. Inhalation of ZnO Nanoparticles
5.5. Animals after the Inhalation and Intratracheal Instillation Studies
5.6. Analysis of Inflammatory Cells in BALF
5.7. Chemokines, LDH, and HO-1 in BALF
5.8. Histopathology
6. TEM Experimental Methods
Statistical Analysis
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, I.S.; Baek, M.; Choi, S.J. Comparative cytotoxicity of Al2O3, CeO2, TiO2 and ZnO nanoparticles to human lung cells. J. Nanosci. Nanotechnol. 2010, 10, 3453–3458. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Fazlollahi, F.; Kennedy, I.M.; Yakobi, N.R.; Hamm-Alvarez, S.F.H.; Borok, Z.; Kim, K.J.; Crandall, E.D. Alveolar epithelial cell injury due to zinc oxide nanoparticle exposure. Am. J. Respir. Crit. Care Med. 2010, 182, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, W.; Zhang, R.; Liu, P.; Wang, Q.; Shang, Y.; Wu, M.; Donaldson, K.; Wang, Q. Comparison of cellular toxicity caused by ambient ultrafine particles and engineered metal oxide nanoparticles. Part Fibre Toxicol. 2015, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Horie, M.; Endoh, S.; Kato, H.; Fujita, K.; Nishio, K.; Komaba, L.K.; Maru, J.; Miyauhi, A.; Nakamura, A.; et al. Association of zinc ion release and oxidative stress induced by intratracheal instillation of ZnO nanoparticles to rat lung. Chem. Biol. Interact. 2012, 198, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Wu, K.Y.; Chein, H.M.; Chen, L.C.; Cheng, T.J. Pulmonary toxicity of inhaled nanoscale and fine zinc oxide particles: Mass and surface area as an exposure metric. Inhal. Toxicol. 2011, 23, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, D.; Yang, H.; Zhang, H.; Zhang, W.; Fang, Y.; Lin, Z.; Tian, L.; Yan, J.; Xi, Z. Comparative study of respiratory tract immune toxicity induced by three sterilization nanoparticles: Silver, zinc oxide and titanium dioxide. J. Hazard Mater. 2013, 248–249, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.; Driscoll, K. Particles, inflammation and respiratory tract carcinogenesis. Toxicol Lett. 1996, 88, 109–113. [Google Scholar] [PubMed]
- Nishi, K.; Morimoto, Y.; Ogami, A.; Murakami, M.; Myojo, T.; Oyabu, T.; Kadoya, C.; Yamamoto, M.; Todoroki, M.; Hirohashi, M.; et al. Expression of cytokine-induced neutrophil chemoattractant in rat lungs by intratracheal instillation of nickel oxide nanoparticles. Inhal. Toxicol. 2009, 21, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer. Oncology 2002, 16, 217–232. [Google Scholar] [PubMed]
- Langley, R.J.; Kalra, R.; Mishra, N.C.; Hahn, F.F.; Razani-Boroujerdi, S.; Singh, S.; Benson, J.M.; Pefia-Philippides, J.C.; Barr, E.B.; Sopori, M.L. A biphasic response to silica. I. Immunostimulation is restricted to the early stage of silicosis in Lewis rats. Am. J. Respir. Cell Mol. Biol. 2004, 30, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Sellamuthu, R.; Umbright, C.; Roberts, J.R.; Chapman, R.; Young, S.H.; Richardson, D.; Leonard, H.; McKinney, W.; Chen, B.; Frazer, D.; et al. Blood gene expression profiling detects silica exposure and toxicity. Toxicol. Sci. 2011, 122, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Konduru, N.V.; Murdaugh, K.M.; Sotiriou, G.A.; Donaghey, T.C.; Demokritou, P.; Brain, J.D.; Molina, R.M. Bioavailability, distribution and clearance of tracheally-instilled and gavages uncoated or silica-coated zinc oxide nanoparticles. Part Fibre Toxicol. 2014, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Adamcakova-Dodd, A.; Stebounova, LV.; Kim, J.S.; Vorrink, S.U.; Ault, A.P.; O’Shaughnessy, P.T.; Grassian, V.H.; Thorne, P.S. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part Fibre Toxicol. 2014, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Akhtar, M.J.; Alhadiag, H.A.; Alrokayan, S.A. Assessment of the lung toxicity of copper oxide nanoparticles: Current status. Nanomedicine 2015, 10, 2365–2377. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Hirohashi, M.; Ogami, A.; Oyabu, T.; Myojo, T.; Nishi, K.; Kadoya, C.; Todoroki, M.; Yamamoto, M.; Murakami, M.; et al. Inflammogenic effect of well-characterized fullerenes in inhalation and intratracheal instillation studies. Part Fibre Toxicol. 2010, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Lee, B.W.; Okada, T.; Oyabu, T.; Myojo, T.; Yatera, K.; Shimada, M.; et al. Comparison of pulmonary inflammatory responses following intratracheal instillation and inhalation nanoparticles. Nanotoxicology 2016, 10, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Fujishima, K.; Yatera, K.; Yamamoto, K. Usefulness of intratracheal instillation studies for estimating nanoparticle-induced pulmonary toxicity. Int. J. Mol. Sci. 2016, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Fubini, B.; Hubbard, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef]
- Schins, R.P. Mechanisms of genotoxicity of particles and fibers. Inhal. Toxicol. 2002, 14, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Kuempel, E.D.; Tran, C.L.; Castranova, V.; Bailer, A.J. Lung dosimetry and risk assessment of nanoparticles: Evaluating and extending current models in rats and humans. Inhal. Toxicol. 2006, 18, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Baisch, B.L.; Corson, N.M.; Wade-Mercer, P.; Gelein, R.; Kennell, A.J.; Obertorster, G.; Elder, A. Equivalent titanium dioxide nanoparticle deposition by intratacheal instillation and whole body inhalation: The effect of dose rate on acute respiratory tract inflammation. Part Fibre Toxicol. 2014, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Hirohashi, M.; Ogami, A.; Oyabu, T.; Myojo, T.; Todoroki, M.; Yamamoto, M.; Hashiba, M.; Mizuguchi, Y.; Lee, B.W.; et al. Pulmonary toxicity of well-dispersed multi-wall carbon nanotubes following inhalation and intratracheal instillation. Nanotoxicology 2012, 6, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; Shimada, M.; Kubo, M.; et al. Pulmonary toxicity of well-dispersed cerium oxide nanoparticles following intratracheal instillation and inhalation. J. Nanopart. Res. 2015, 17, 442. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.M.; Doudrick, K.; Franzi, L.M.; TeeSy, C.; Anderson, D.S.; Wu, Z.; Mitra, S.; Vu, V.; Dutrow, G.; Evans, J.E.; et al. Instillation versus inhalation of multiwalled carbon nanotubes: Exposure-related health effects, clearance, and the role of particle characteristics. ACS Nano 2014, 8, 8911–8931. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.H.; Chuang, K.J.; Chen, J.K.; Hsiao, T.C.; Lai, C.H.; Jones, T.P.; Berube, K.A.; Hong, G.B.; Ho, K.F.; Chuang, H.C. Characterization of pulmonary protein profiles in response to zinc oxide nanoparticles in mice: A 24-hour and 28-day follow-up study. Int. J. Nanomed. 2015, 10, 4705–4716. [Google Scholar]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Unitrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Oyabu, T.; Morimoto, Y.; Hirohashi, M.; Horie, M.; Kambara, T.; Lee, B.W.; Hashiba, M.; Mizuguchi, Y.; Myojo, T.; Kuroda, E. Dose-dependent pulmonary response of well-dispersed titanium dioxide nanoparticles following intratracheal instillation. J. Nanopart. Res. 2013, 15, 1600. [Google Scholar] [CrossRef]
- Kubo, M.; Nakaoka, A.; Morimoto, K.; Shimada, M.; Horie, M.; Morimoto, Y.; Sasaki, T. Aerosol generation by a spray-drying technique under coulomb explosion and rapid evaporation for the preparation of aerosol particles for inhalation tests. Aerosol Sci. Technol. 2014, 48, 698–705. [Google Scholar] [CrossRef]
- Shimada, M.; Wang, W.N.; Okuyama, K.; Myojo, T.; Oyabu, T.; Morimoto, Y.; Tanaka, I.; Endoh, S.; Uchida, K.; Ehara, K.; et al. Development and evaluation of an aerosol generation and supplying system for inhalation experiments of manufactured nanoparticles. Environ. Sci. Technol. 2009, 43, 5529–5534. [Google Scholar] [CrossRef] [PubMed]








| Time | 3 Days (n = 5) | 1 Week (n = 5) | 1 Month (n = 5) | 3 Months (n = 5) | 6 Months (n = 5) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathological Feature | Negative Control | ZnO 0.2 mg | ZnO 1.0 mg | Negative Control | ZnO 0.2 mg | ZnO 1.0 mg | Negative Control | ZnO 0.2 mg | ZnO 1.0 mg | Negative Control | ZnO 0.2 mg | ZnO 1.0 mg | Negative Control | ZnO 0.2 mg | ZnO 1.0 mg |
| Macrophage infiltration in alveolar space | − | ++ | ++ | − | + | + | − | ± | ± | − | − ~ ± | − ~ ± | − | − ~ ± | − ~ ± |
| Inflammatory cell infiltration in alveolar space | − | ++ | +++ | − | + | + | − | − | − ~ ± | − | − | − | − | − | − |
| Infiltration in interstitial area | − | + | ++ | − | ± | ± | − | − | − ~ ± | − | − | − | − | − | − |
| Hyperplasia of bronchiolar epithelial cell | − | + | + | − | − ~ ± | − ~ ± | − | − ~ ± | − ~ ± | − | − | − ~ ± | − | − | − ~ ± |
| Hyperplasia of alveolar epithelial cell | − | ++ | ++ ~ +++ | − | ± | ± | − | − | − | − | − | − | − | − | − |
| Fibrosis | − | ± ~ + | ± ~ + | − | ± | ± | − | − | − ~ ± | − | − | − ~ ± | − | − | − ~ ± |
| Tumor | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Time | 3 Days (n = 5) | 1 Month (n = 5) | 3 Months (n = 5) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pathological Feature | Negative Control | ZnO Low | ZnO High | Negative Control | ZnO Low | ZnO High | Negative Control | ZnO Low | ZnO High |
| Macrophage infiltration in alveolar space | − | + | ++ | − | ± | + | − | ± | ± |
| Inflammatory cell infiltration in alveolar space | − | − | − ~ ± | − | − | − | − | − | − |
| Infiltration in interstitial area | − | − | − ~ ± | − | − | − | − | − | − |
| Hyperplasia of bronchiolar epithelial cell | − | − | − ~ ± | − | − | − | − | − | − |
| Hyperplasia of alveolar epithelial cell | − | − | − | − | − | − | − | − | − |
| Fibrosis | − | − | − | − | − | − | − | − | − |
| tumor | − | − | − | − | − | − | − | − | − |
| Nanomaterials | ZnO Nanoparticle |
|---|---|
| Manufacturer | Sigma-Aldrich Co. LLC. |
| Chemical formula | ZnO |
| Primary diameter | 35 nm |
| Specific surface area | 31 m2/g |
| Shape | Polyhedral roughly round |
| Secondary diameter (DLS) | 33 nm |
| Purity | 99.94 wt % |
| Bulk density | 5.6 g/cm3 |
| Solubility | high |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; Shimada, M.; Kubo, M.; et al. Evaluation of Pulmonary Toxicity of Zinc Oxide Nanoparticles Following Inhalation and Intratracheal Instillation. Int. J. Mol. Sci. 2016, 17, 1241. https://doi.org/10.3390/ijms17081241
Morimoto Y, Izumi H, Yoshiura Y, Tomonaga T, Oyabu T, Myojo T, Kawai K, Yatera K, Shimada M, Kubo M, et al. Evaluation of Pulmonary Toxicity of Zinc Oxide Nanoparticles Following Inhalation and Intratracheal Instillation. International Journal of Molecular Sciences. 2016; 17(8):1241. https://doi.org/10.3390/ijms17081241
Chicago/Turabian StyleMorimoto, Yasuo, Hiroto Izumi, Yukiko Yoshiura, Taisuke Tomonaga, Takako Oyabu, Toshihiko Myojo, Kazuaki Kawai, Kazuhiro Yatera, Manabu Shimada, Masaru Kubo, and et al. 2016. "Evaluation of Pulmonary Toxicity of Zinc Oxide Nanoparticles Following Inhalation and Intratracheal Instillation" International Journal of Molecular Sciences 17, no. 8: 1241. https://doi.org/10.3390/ijms17081241