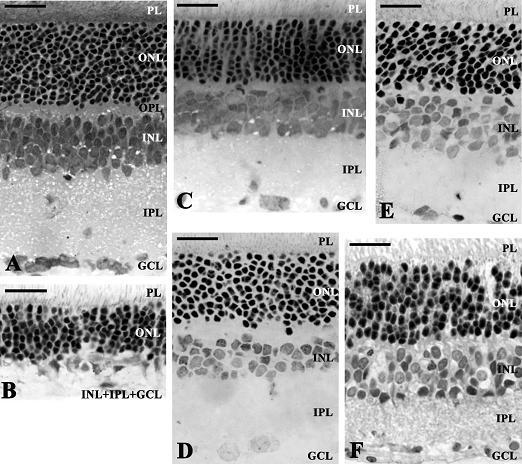
Novel Neuroprotective Strategies in Ischemic Retinal Lesions
Abstract
:1. Retinal Ischemia
2. Potential Protective Strategies
3. Urocortin 2
4. Diazoxide
5. Pituitary Adenylate Cyclase Activating Polypeptide
6. PARP Inhibition
7. Conclusions
Acknowledgments
References
- Osborne, NN; Casson, RJ; Wood, JPM; Chidlow, G; Graham, M; Melena, J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res 2004, 23, 91–147. [Google Scholar]
- Harris, A; Jonescu-Cuypers, CP; Kagemann, L; Krieglstein, GK. Atlas of Ocular Blood Flow–Vascular Anatomy, Pathophysiology, and Metabolism; Imprint of Butterworth Heinemann: Philadelphia, PA, USA, 2003. [Google Scholar]
- Feigl, B. Age-related maculopathy-linking aetiology and pathophysiologycal changes to the ischaemia hypothesis. Prog. Retin. Eye Res 2009, 28, 63–86. [Google Scholar]
- Kaur, C; Foulds, WS; Ling, EA. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog. Retin. Eye Res 2008, 27, 622–647. [Google Scholar]
- Pournaras, CJ; Rungger-Brandle, E; Riva, CE; Hardarson, SH; Stefansson, E. Regulation of retinal blood flow in health and disease. Prog. Retin. Eye Res 2008, 27, 284–330. [Google Scholar]
- Chen, CS; Miller, NR. Ocular ischemic syndrome: review of clinical presentations, etiology, investigation, and management. Compr. Ophthalmol 2007, 8, 17–28. [Google Scholar]
- Osborne, NN; Ugarte, M; Chao, M; Chidlow, G; Bae, JH; Wood, JP; Nash, MS. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv. Ophthalmol 1999, 1, 102–128. [Google Scholar]
- Bek, T. Inner retinal ischemia: current understanding and needs for further investigations. Acta Ophthalmol 2009, 87, 362–367. [Google Scholar]
- Roth, S. Endogenous neuroprotection in the retina. Brain Res. Bull 2004, 62, 461–466. [Google Scholar]
- Fulton, AB; Akula, JD; Mocko, JA; Hansen, RM; Benador, IY; Beck, SC; Fahl, E; Seeliger, MW; Moskowitz, A; Harris, ME. Retinal degenerative and hypoxic ischemic disease. Doc. Ophthalmol 2009, 118, 55–61. [Google Scholar]
- Kalesnykas, G; Tuulos, T; Uusitalo, H; Jolkkonen, J. Neurogenereration and cellular stress in the retina and optic nerve in rat cerebral ischemia and hypoperfusion models. Neuroscience 2008, 55, 937–947. [Google Scholar]
- Farkas, E; Luiten, PG; Bari, F. Permanent, bilateral common carotid artery occlusion in the rat: A model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res. Rev 2007, 54, 162–180. [Google Scholar]
- Slakter, JS; Spertus, AD; Weissman, SS; Henkind, P. An experimental model of carotid artery occlusive disease. Am. J. Ophtalmol 1984, 97, 168–172. [Google Scholar]
- Spertus, AD; Slakter, JS; Weissman, SS; Henkind, P. Experimental carotid occlusion: funduscopic and fluorescein angiographic findings. Br. J. Ophtalmol 1984, 68, 47–57. [Google Scholar]
- Block, F; Schwarz, M; Sontag, KH. Retinal ischemia induced by occlusion of both common carotid arteries in rats as demonstrated by electroretinography. Neurosci. Lett 1992, 144, 124–126. [Google Scholar]
- Osborne, NN; Safa, R; Nash, MS. Photoreceptors are preferentially affected in the rat retina following permanent occlusion of the carotid arteries. Vision Res 1999, 39, 3995–4002. [Google Scholar]
- Stevens, WD; Fortin, T; Pappas, BA. Retinal and optic nerve degeneration after chronic carotid ligation. Stroke 2002, 33, 1107–1112. [Google Scholar]
- Yamamoto, H; Schmidt-Kasner, R; Hamasaki, DI; Yamamoto, H; Parel, JM. Complex neurodegeneration in retina following moderate ischemia induced by bilateral common carotid artery occlusion in Wistar rats. Exp. Eye Res 2006, 82, 767–779. [Google Scholar]
- Lavinsky, D; Arterni, NS; Achaval, M; Netto, CA. Chronic bilateral common carotid artery occlusion: a model for ocular ischemic syndrome in the rat. Graefe’s Arch. Clin. Exp. Ophthalmol 2006, 244, 199–204. [Google Scholar]
- Mester, L; Szabo, A; Atlasz, T; Szabadfi, K; Reglodi, D; Kiss, P; Racz, B; Tamas, A; Gallyas, F; Sumegi, B; Hocsak, E; Gabriel, R; Kovacs, K. Protection against chronic hypoperfusion-induced retinal neurodegeneration by PARP inhibition via activation of PI3-kinase Akt pathway and suppression of JNK and p38 MAP kinases. Neurotox. Res 2009, 18, 68–76. [Google Scholar]
- Atlasz, T; Babai, N; Kiss, P; Reglodi, D; Tamas, A; Szabadfi, K; Toth, G; Hegyi, O; Lubics, A; Gabriel, R. Pituitary adenylate cyclase activating polypeptide is protective in bilateral carotid occlusion-induced retinal lesion in rats. Gen. Comp. Endocrinol 2007, 153, 108–114. [Google Scholar]
- Vidal-Sanz, M; Lafuente, M; Sobrado-Calvo, P; Selles-Navarro, I; Rodriguez, E; Mayor-Torroglosa, S; Villegas-Perez, MP. Death and neuroprotection of retinal ganglion cells after different types of injury. Neurotox. Res 2000, 2, 215–227. [Google Scholar]
- Dilsiz, N; Sahaboglu, A; Yildiz, MZ; Reichenbach, A. Protective effects of various antioxidants during ischemia-reperfusion in the rat retina. Graefe’s Arch. Clin. Exp. Ophthalmol 2006, 244, 627–633. [Google Scholar]
- Li, SY; Fu, ZJ; Ma, H; Jang, WC; So, KF; Wong, D; Lo, AC. Effect of lutein on retinal neurons and oxidative stress in a model of acute retinal ischemia/reperfusion. Invest. Ophthalmol. Vis. Sci 2009, 50, 836–843. [Google Scholar]
- Maher, P; Hanneken, A. Flavonoids protect retinal ganglion cells from ischemia in vitro. Exp. Eye Res 2008, 86, 366–374. [Google Scholar]
- Roth, S; Li, B; Rosenbaum, PS; Gupta, H; Goldstein, IM; Maxwell, KM; Gidday, JM. Preconditioning provides complete protection against retinal ischemic injury in rats. Invest. Ophthalmol. Vis. Sci 1998, 39, 777–785. [Google Scholar]
- Obolensky, A; Berenshtein, E; Konijn, AM; Banin, E; Chevion, M. Ischemic preconditioning of the rat retina: protective role of ferritin. Free Radic. Biol. Med 2008, 44, 1286–1294. [Google Scholar]
- Sakamoto, K; Yonoki, Y; Kuwagata, M; Saito, M; Nakahara, T; Ishii, K. Histological protection against ischemia-reperfusion injury by early ischemic preconditioning in rat retina. Brain Res 2004, 1015, 154–160. [Google Scholar]
- Chollangi, S; Wang, J; Martin, A; Quinn, J; Ash, JD. Preconditioning-induced protection from oxidative injury is mediated by leukemia inhibitory factor receptor (LIFR) and its ligands in the retina. Neurobiol. Dis 2009, 34, 535–544. [Google Scholar]
- Li, Y; Roth, S; Laser, M; Ma, JX; Crosson, CE. Retinal preconditioning and the induction of heat-shock protein 27. Invest. Ophthalmol. Vis. Sci 2003, 44, 1299–1304. [Google Scholar]
- Fernandez, DC; Chianelli, MS; Rosenstein, RE. Involvement of glutamate in retinal protection against ischemia/reperfusion damage induced by post-conditioning. J. Neurochem 2009, 111, 488–498. [Google Scholar]
- Macaluso, C; Frishman, LJ; Frueh, B; Kaelin-Lang, A; Onoe, S; Niemeyer, G. Multiple effects of adenosine in the arterially perfused mammalian eye. Possible mechanisms for the neuroprotective function of adenosine in the retina. Doc. Ophthalmol 2003, 106, 51–59. [Google Scholar]
- Tomita, H; Ishiguro, S; Abe, T; Tamai, M. Administration of nerve growth factor, brain-derived neurotrophic factor and insulin-like growth factor-II protects phosphate-activated glutaminase in the ischemic and reperfused rat retinas. Tohoku J. Exp. Med 1999, 187, 227–236. [Google Scholar]
- Nishijima, K; Ng, Y-S; Zhong, L; Bradley, J; Schubert, W; Jo, N; Akita, J; Samuelsson, SJ; Robinson, GS; Adamis, AP; Shima, DT. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am. J. Pathol 2007, 171, 53–67. [Google Scholar]
- Sivilia, S; Giuliani, A; Fernández, M; Turba, ME; Forni, M; Massella, A; de Sordi, N; Giardino, L; Calzà, L. Intravitreal NGF administration counteracts retina degeneration after permanent carotid artery occlusion in rat. BMC Neurosci 2009, 10, 52. [Google Scholar]
- Junk, AK; Mammis, A; Savitz, SI; Singh, M; Roth, S; Malhotra, S; Rosenbaum, PS; Cerami, A; Brines, M; Rosenbaum, DM. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2002, 99, 10659–10664. [Google Scholar]
- Dreixler, JC; Hagevik, S; Hemmert, JW; Shaikh, AR; Rosenbaum, DM; Roth, S. Involvement of erythropoietin in retinal ischemic preconditioning. Anesthesiology 2009, 110, 774–780. [Google Scholar]
- Jehle, T; Meschede, W; Dersch, R; Feltgen, N; Bach, M; Lagreze, WA. Erythropoietin protects retinal ganglion cells and visual function after ocular ischemia and optic nerve compression. Ophthalmologe 2009, in press. [Google Scholar]
- Schmeer, C; Gamez, A; Tausch, S; Witte, OW; Isenmann, S. Statins modulate heat shock protein expression and enhance retinal ganglion cell survival after transient retinal ischemia/reperfusion in vivo. Invest. Ophthalmol. Vis. Sci 2008, 49, 4971–4981. [Google Scholar]
- Russo, R; Cavaliere, F; Watanabe, C; Nucci, C; Bagetta, G; Corasaniti, MT; Sakurada, S; Morrone, LA. 17Beta-estradiol prevents retinal ganglion cell loss induced by acute rise of intraocular pressure in rat. Prog. Brain Res 2008, 173, 583–590. [Google Scholar]
- El-Remessy, AB; Khalil, IE; Matragoon, S; Abou-Mohamed, G; Tsai, NJ; Roon, P; Caldwell, RB; Caldwell, RW; Green, K; Liou, GI. Neuroprotective effect of (−)Delta9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: involvement of peroxynitrite. Am. J. Pathol 2003, 163, 1997–2008. [Google Scholar]
- Riazi-Esfahani, M; Kiumehr, S; Asadi-Amoli, F; Lashay, AR; Dehpour, AR. Morphine pretreatment provides histologic protection against ischemia-reperfusion injury in rabbit retina. Retina 2008, 28, 511–517. [Google Scholar]
- Riazi-Esfahani, M; Kiumehr, S; Asadi-Amoli, F; Dehpour, AR. Effects of intravitreal morphine administered at different time points after reperfusion in a rabbit model of ischemic retinopathy. Retina 2009, 29, 262–268. [Google Scholar]
- Kocer, I; Kulacoglu, D; Altuntas, I; Gundogdu, C; Gullulu, G. Protection of the retina from ischemia-reperfusion injury by L-carnitine in guinea pigs. Eur. J. Ophthalmol 2003, 13, 80–85. [Google Scholar]
- Lombardi, G; Moroni, F. Glutamate receptor antagonists protect against ischemia-induced retinal damage. Eur. J. Pharmacol 1994, 271, 489–495. [Google Scholar]
- Wood, JP; Schmidt, KG; Melena, J; Chidlow, G; Allmeier, H; Osborne, NN. The beta-adrenoceptor antagonists metipranolol and timolol are retinal neuroprotectants: comparison with betaxolol. Exp. Eye Res 2003, 76, 505–516. [Google Scholar]
- Donello, JE; Padillo, EU; Webster, ML; Wheeler, LA; Gil, DW. Alpha(2)-Adrenoceptor agonists inhibit vitreal glutamate and aspartate accumulation and preserve retinal function after transient ischemia. J. Pharmacol. Exp. Ther 2001, 296, 216–223. [Google Scholar]
- Ettaiche, M; Heurteaux, C; Blondeau, N; Borsotto, M; Tinel, N; Lazdunski, M. ATP-sensitive potassium channels [K(ATP)] in retina: a key role for delayed ischemic tolerance. Brain Res 2001, 890, 118–129. [Google Scholar]
- Sakamoto, K; Kawakami, T; Shimada, M; Yamaguchi, A; Kuwagata, M; Saito, M; Nakahara, T; Ishii, K. Histological protection by cilnidipine, a dual L/N-type Ca(2+) channel blocker, against neurotoxicity induced by ischemia-reperfusion in rat retina. Exp. Eye Res 2009, 88, 974–982. [Google Scholar]
- Traustason, S; Eysteinsson, T; Agnarsson, BA; Stefánsson, E. GABA agonists fail to protect the retina from ischemia-reperfusion injury. Exp. Eye Res 2009, 88, 361–366. [Google Scholar]
- Holman, MC; Chidlow, G; Wood, JP; Casson, RJ. Hyperglycemia rescues retinal neurons from hypoperfusion-induced injury. Invest Ophthalmol Vis Sci 2009, in press. [Google Scholar]
- Fekete, ÉM; Zorrilla, EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: Ancient CRF paralogs. Front. Neuroendocrinol 2007, 28, 1–27. [Google Scholar]
- Chen, A; Perrin, M; Brar, B; Li, C; Jamieson, P; Digruccio, M; Lewis, K; Vale, W. Mouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol. Endocrinol 2005, 19, 441–458. [Google Scholar]
- Latchman, DS. Molecules in focus urocortin. Biochem. Cell Biol 2002, 34, 907–910. [Google Scholar]
- Pan, W; Kastin, AJ. Urocortin and the brain. Prog. Neurobiol 2008, 84, 148–156. [Google Scholar]
- Skelton, KH; Owens, MJ; Nemeroff, CB. The neurobiology of urocortin. Regul. Pept 2000, 93, 85–92. [Google Scholar]
- Tsatsanis, C; Androulidaki, A; Dermitzaki, E; Charalampopoulos, I; Spiess, J; Gravanis, A; Margioris, AN. Urocortin 1 and Urocortin 2 induce macrophage apoptosis via CRFR2. FEBS Lett 2005, 579, 4259–4264. [Google Scholar]
- Uchida, M; Suzuki, M; Shimizu, K. Effects of urocortin, corticotropin-releasing factor (CRF) receptor agonist, and astressin, CRF receptor antagonist, on the sleep-wake pattern: analysis by radiotelemetry in conscious rats. Biol. Pharm. Bull 2007, 10, 1895–1897. [Google Scholar]
- Brar, BK; Jonassen, AK; Egorina, EM; Chen, A; Negro, A; Perrin, MH; Mjøs, OD; Latchman, DS; Lee, KF; Vale, W. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology 2004, 145, 24–35. [Google Scholar]
- Liu, CN; Yang, C; Liu, XY; Li, S. In vivo protective effects of urocortin on ischemiareperfusion injury in rat heart via free radical mechanisms. Can. J. Physiol. Pharmacol 2005, 83, 459–465. [Google Scholar]
- Rademaker, MT. Urocortin: cardiovascular actions and therapeutic implications. Lett. Drug Des. Discov 2004, 1, 168–172. [Google Scholar]
- Tao, J; Li, S. Effects of UCN via ion mechanisms or CRF receptors? Biochem. Biophys. Res. Commun 2005, 336, 731–736. [Google Scholar]
- Facci, L; Stevens, DA; Pangallo, M; Franceschini, D; Skaper, SD; Strijbos, PJLM. Corticotropin-releasing factor (CRF) and related peptides confer neuroprotection via type 1 CRF receptors. Neuropharmacol 2003, 45, 623–636. [Google Scholar]
- Pedersen, WA; Wan, R; Zhang, P; Mattson, MP. Urocortin, but not urocortin II, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type I. J. Neurosci 2002, 22, 404–412. [Google Scholar]
- Dautzenberg, FM; Huber, G; Higelin, J; Py-Lang, G; Kilpatrick, GJ. Evidence for the abundant expression of arginine 185 containing human CRF2 receptors and the role of position 185 for receptor-ligand selectivity. Neuropharmacol 2000, 39, 1368–1376. [Google Scholar]
- Zmijewski, MA; Sharma, RK; Slominski, AT. Expression of molecular equivalent of hypothalamic-pituitary-adrenal axis in adult retinal pigment epithelium. J. Endocrinol 2007, 193, 157–169. [Google Scholar]
- Skofitsch, G; Jacobowitz, DM. Corticotropin releasing factor-like immunoreactive neurons in the rat retina. Brain Res. Bull 1984, 12, 539–542. [Google Scholar]
- Williamson, DE; Eldred, WD. Synaptic organization of two types of amacrine cells with CRF-like immunoreactivity in the turtle retina. Vis. Neurosci 1991, 6, 257–269. [Google Scholar]
- Williamson, DE; Eldred, WD. Amacrine and ganglion cells with corticotropinreleasing-factor-like immunoreactivity in the turtle retina. J. Comp. Neurol 1989, 280, 424–435. [Google Scholar]
- Zhang, DR; Gallagher, M; Sladek, CD; Yeh, HH. Postnatal development of corticotrophin releasing factor-like immunoreactive amacrine cells in the rat retina. Brain Res. Dev. Brain Res 1990, 51, 185–194. [Google Scholar]
- Zhang, DR; Yeh, HH. Corticotropin releasing factor-like immunoreactivity (CRFLI) in horizontal cells of the developing rat retina. Vis. Neurosci 1991, 6, 383–391. [Google Scholar]
- Zhang, DR; Yeh, HH. Histogenesis of corticotropin releasing factor-like immunoreactive amacrine cells in the rat retina. Brain Res. Dev. Brain Res 1990, 53, 194–199. [Google Scholar]
- Szabadfi, K; Atlasz, T; Reglodi, D; Kiss, P; Danyáadi, B; Fekete, ÉM; Zorrilla, EP; Tamas, A; Szabo, K; Gabriel, R. Urocortin 2 protects against retinal degeneration following bilateral common carotid artery occlusion in the rat. Neurosci. Lett 2009, 455, 42–45. [Google Scholar]
- Busija, DW; Lacza, Z; Rajapakse, N; Shimizu, K; Kis, B; Bari, F; Domoki, F; Horiguchi, T. Targeting mitochondrial ATP-sensitive potassium channels - a novel approach to neuroprotection. Brain Res. Rev 2004, 46, 282–294. [Google Scholar]
- Yamauchi, T; Kashii, S; Yasuyoshi, H; Zhang, S; Honda, Y; Akaike, A. Mitochondrial ATP-sensitive potassium channel: a novel site for neuroprotection. Invest. Ophthalmol. Vis. Sci 2003, 44, 2750–2756. [Google Scholar]
- Domoki, F; Perciaccante, JV; Veltkamp, R; Bari, F; Busija, DW. Mitochondrial potassium channel opener diazoxide preserves neuronal-vascular function after cerebral ischemia in newborn pigs. Stroke 1999, 30, 2713–2718. [Google Scholar]
- Liu, D; Lu, C; Wan, R; Auyeung, WW; Mattson, MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J. Cereb. Blood Flow Metab 2002, 22, 431–443. [Google Scholar]
- Minners, J; McLeod, CJ; Sack, MN. Mitochondrial plasticity in classical ischemic preconditioning-moving beyond the mitochondrial KATP channel. Cardiovasc. Res 2003, 59, 1–6. [Google Scholar]
- Shake, JG; Peck, EA; Marban, E; Gott, VL; Johnston, MV; Troncoso, JC; Redmond, JM; Baumgartner, WA. Pharmacologically induced preconditioning with diazoxide: a novel approach to brain protection. Ann. Thorac. Surg 2001, 72, 1849–1854. [Google Scholar]
- Nagy, K; Kis, B; Rajapakse, NC; Bari, F; Busija, DW. Diazoxide preconditioning protects against neuronal cell death by attenuation of oxidative stress upon glutamate stimulation. J. Neurosci. Res 2004, 76, 697–704. [Google Scholar]
- Teshima, Y; Akao, M; Li, RA; Chong, TH; Baumgartner, WA; Johnston, MV; Marban, E. Mitochondrial ATP-sensitive potassium channel activation protects cerebellar granule neurons from apoptosis induced by oxidative stress. Stroke 2003, 34, 1796–1802. [Google Scholar]
- Rajapakse, N; Kis, B; Horiguchi, T; Snipes, J; Busija, DW. Diazoxide pretreatment induces delayed preconditioning in astrocytes against oxygen glucose deprivation and hydrogen peroxide-induced toxicity. J. Neurosci. Res 2003, 73, 206–214. [Google Scholar]
- Domoki, F; Bari, F; Nagy, K; Busija, DW; Siklós, L. Diazoxide prevents mitochondrial swelling and Ca2+ accumulation in CA1 pyramidal cells after cerebral ischemia in newborn pigs. Brain Res 2004, 1019, 97–104. [Google Scholar]
- Kis, B; Rajapakse, NC; Snipes, JA; Nagy, K; Horiguchi, T; Busija, DW. Diazoxide induces delayed pre-conditioning in cultured rat cortical neurons. J. Neurochem 2003, 87, 969–980. [Google Scholar]
- Liu, Y; Sato, T; Seharaseyon, J; Szewczyk, A; O’Rourke, B; Marban, E. Mitochondrial ATP-dependent potassium channels. Viable candidate effectors of ischemic preconditioning. Ann. NY Acad. Sci 1999, 874, 27–37. [Google Scholar]
- Shimizu, K; Lacza, Z; Rajapakse, N; Horiguchi, T; Snipes, J; Busija, DW. MitoK(ATP) opener, diazoxide, reduces neuronal damage after middle cerebral artery occlusion in the rat. Am. J. Physiol. Heart Circ. Physiol 2002, 283, 1005–1011. [Google Scholar]
- Busija, DW; Katakam, P; Rajapakse, NC; Kis, B; Grover, G; Domoki, F; Bari, F. Effects of ATP-sensitive potassium channel activators diazoxide and BMS-191095 on membrane potential and reactive oxygen species production in isolated piglet mitochondria. Brain Res. Bull 2005, 66, 85–90. [Google Scholar]
- Farkas, E; Annahazi, A; Institoris, A; Mihaly, A; Luiten, PG; Bari, F. Diazoxide and dimethyl sulphoxide alleviate experimental cerebral hypoperfusion-induced white matter injury in the rat brain. Neurosci. Lett 2005, 373, 195–199. [Google Scholar]
- Farkas, E; Timmer, NM; Domoki, F; Mihaly, A; Luiten, PG; Bari, F. Post-ischemic administration of diazoxide attenuates long-term microglial activation in the rat brain after permanent carotid artery occlusion. Neurosci. Lett 2005, 387, 168–172. [Google Scholar]
- Lenzser, G; Kis, B; Bari, F; Busija, DW. Diazoxide preconditioning attenuates global cerebral ischemia-induced blood-brain barrier permeability. Brain Res 2005, 1051, 72–80. [Google Scholar]
- Farkas, E; Institoris, A; Domoki, F; Mihaly, A; Luiten, PG; Bari, F. Diazoxide and dimethyl sulphoxide prevent cerebral hypoperfusion-related learning dysfunction and brain damage after carotid artery occlusion. Brain Res 2004, 1008, 252–260. [Google Scholar]
- Farkas, E; Institoris, A; Domoki, F; Mihaly, A; Bari, F. The effect of pre- and posttreatment with diazoxide on the early phase of chronic cerebral hypoperfusion in the rat. Brain Res 2006, 1087, 168–174. [Google Scholar]
- Sheu, SJ; Wu, SN. Mechanism of inhibitory actions of oxidizing agents on calcium-activated potassium current in cultured pigment epithelial cells of the human retina. Invest. Ophthalmol. Vis. Sci 2003, 44, 1237–1244. [Google Scholar]
- Pielen, A; Kirsch, M; Hofmann, HD; Feuerstein, TJ; Lagreze, WA. Retinal ganglion cell survival is enhanced by gabapentin-lactam in vitro: evidence for involvement of mitochondrial KATP channels. Graefe’s Arch. Clin. Exp. Ophthalmol 2004, 242, 240–244. [Google Scholar]
- Hankins, MW; Ikeda, H. Consequences of transient retinal hypoxia on rod input to horizontal cells in the rat retina. Vision Res 1993, 33, 429–436. [Google Scholar]
- Roth, S; Dreixler, JC; Shaikh, AR; Lee, KH; Bindokas, V. Mitochondrial potassium ATP channels and retinal ischemic preconditioning. Invest. Ophthalmol. Vis. Sci 2006, 47, 2114–2124. [Google Scholar]
- Jehle, T; Lagreze, WA; Blauth, E; Knorle, R; Schnierle, P; Lucking, CH; Feuerstein, TJ. Gabapentin-lactam (8-aza-spiro[5,4]decan-9-on; GBP-L) inhibits oxygen glucose deprivation-induced [3H]glutamate release and is a neuroprotective agent in a model of acute retinal ischemia. Naunyn Schmiedebergs Arch. Pharmacol 2000, 362, 74–81. [Google Scholar]
- Atlasz, T; Babai, N; Reglodi, D; Kiss, P; Tamas, A; Bari, F; Domoki, F; Gabriel, R. Diazoxide is protective in the rat retina against ischemic injury induced by bilateral carotid occlusion and glutamate-induced degeneration. Neurotox. Res 2007, 12, 105–111. [Google Scholar]
- Unoki, K; La Vail, MM. Protection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest. Ophtalmol. Vis. Sci 1994, 35, 907–915. [Google Scholar]
- Ogata, N; Wang, L; Jo, N; Tombran-Tink, J; Takahashi, K; Mrazek, D; Matsumura, M. Pigment epithelium derived factor as a neuroprotective agent against ischemic retinal injury. Curr. Eye Res 2001, 22, 245–252. [Google Scholar]
- Shibuki, H; Katai, N; Kuroiwa, S; Kurokawa, T; Arai, J; Matsumoto, K; Nakamura, T; Yoshimura, N. Expression and neuroprotective effect of hepatocyte growth factor in retinal ischemia-reperfusion injury. Invest. Ophthalmol. Vis. Sci 2002, 43, 528–536. [Google Scholar]
- Vaudry, D; Falluel-Morel, A; Bourgault, S; Basille, M; Burel, D; Wurtz, O; Fournier, A; Chow, BK; Hashimoto, H; Galas, L; Vaudry, H. Pituitary adenylate cyclase activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev 2009, 61, 283–357. [Google Scholar]
- Ohtaki, H; Nakamachi, T; Dohi, K; Shioda, S. Role of PACAP in ischemic neural death. J. Mol. Neurosci 2008, 36, 16–25. [Google Scholar]
- Somogyvari-Vigh, A; Reglodi, D. Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide-review. Curr. Pharm. Des 2004, 10, 2861–2889. [Google Scholar]
- Seki, T; Izumi, S; Shioda, S; Zhou, CJ; Arimura, A; Koide, R. Gene expression for PACAP receptor mRNA in the rat retina by in situ hybridization and in situ RT-PCR. Ann. N. Y. Acad. Sci 2000, 921, 366–369. [Google Scholar]
- Seki, T; Shioda, S; Ogino, D; Nakai, Y; Arimura, A; Koide, R. Distribution and ultrastructural localization of a receptor for pituitary adenylate cyclase activating polypeptide and its mRNA in the rat retina. Neurosci. Lett 1997, 238, 127–130. [Google Scholar]
- Waschek, JA. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev. Neurosci 2002, 24, 14–23. [Google Scholar]
- Bagnoli, P; Dal Monte, M; Casini, G. Expression of neuropeptides and their receptors in the developing retina of mammals. Histol. Histopathol 2003, 18, 1219–1242. [Google Scholar]
- Borba, JC; Henze, IP; Silveira, MS; Kubrusly, RC; Gardino, PF; de Mello, MC; Hokoc, JN; de Mello, FG. Pituitary adenylate cyclase activating polypeptide (PACAP) can act as determinant of the tyrosine hydoxylase phenotype of dopaminergic cells during retina development. Dev. Brain Res 2005, 156, 193–201. [Google Scholar]
- Shoge, K; Mishima, HK; Saitoh, T; Ishihara, K; Tamura, Y; Shiomi, H; Sasa, M. Attenuation by PACAP of glutamate-induced neurotoxicity in cultured retinal neurons. Brain Res 1999, 839, 66–73. [Google Scholar]
- Silveira, MS; Costa, MR; Bozza, M; Linden, R. Pituitary adenylate cyclase activating polypeptide prevents induced cell death in retinal tissue through activation of cyclic AMP-dependent protein kinase. J. Biol. Chem 2002, 277, 16075–16080. [Google Scholar]
- Rabl, K; Reglodi, D; Banvolgyi, T; Somogyvari-Vigh, A; Lengvari, I; Gabriel, R; Arimura, A. PACAP inhibits anoxia-induced changes in physiological responses in horizontal cells in the turtle retina. Regul. Pept 2002, 109, 71–74. [Google Scholar]
- Seki, T; Itoh, H; Nakamachi, T; Shioda, S. Suppression of ganglion cell death by PACAP following optic nerve transection in the rat. J. Mol. Neurosci 2008, 36, 57–60. [Google Scholar]
- Babai, N; Atlasz, T; Tamas, A; Reglodi, D; Toth, G; Kiss, P; Gabriel, R. Search for the optimal monosodium glutamate treatment schedule to study the neuroprotective effects of PACAP in the retina. Ann. N.Y. Acad. Sci 2006, 1070, 149–155. [Google Scholar]
- Babai, N; Atlasz, T; Tamas, A; Reglodi, D; Toth, G; Kiss, P; Gabriel, R. Degree of damage compensation by various PACAP treatments in monosodium glutamate-induced retinal degeneration. Neurotox. Res 2005, 8, 227–233. [Google Scholar]
- Tamas, A; Gabriel, R; Racz, B; Denes, V; Kiss, P; Lubics, A; Lengvari, I; Reglodi, D. Effects of pituitary adenylate cyclase activating polypeptide in retinal degeneration induced by monosodium-glutamate. Neurosci. Lett 2004, 372, 110–113. [Google Scholar]
- Atlasz, T; Szabadfi, K; Reglodi, D; Kiss, P; Tamas, A; Toth, G; Molnar, A; Szabo, K; Gabriel, R. Effects of pituitary adenylate cyclase activating polypeptide (PACAP1-38) and its fragments on retinal degeneration induced by neonatal MSG treatment. Ann. NY Acad. Sci 2009, 1163, 348–352. [Google Scholar]
- Seki, T; Nakatani, M; Taki, C; Shinonara, Y; Ozawa, M; Nishimura, S; Shioda, S. Neuroprotective effect of PACAP against kainic acid (KA)-induced neurotoxicity in rat retina. Ann. NY Acad. Sci 2006, 1070, 531–534. [Google Scholar]
- Racz, B; Tamas, A; Kiss, P; Toth, G; Gasz, B; Borsiczky, B; Ferencz, A; Gallyas, F, Jr; Roth, E; Reglodi, D. Involvement of ERK and CREB signalling pathways in the protective effect of PACAP on monosodium glutamate-induced retinal lesion. Ann. NY Acad. Sci 2006, 1070, 507–511. [Google Scholar]
- Racz, B; Gallyas, F, Jr; Kiss, P; Toth, G; Hegyi, O; Gasz, B; Borsiczky, B; Ferencz, A; Roth, E; Tamas, A; Lengvari, I; Lubics, A; Reglodi, D. The neuroprotective effects of PACAP in monosodium glutamate-induced retinal lesion involves inhibition of proapoptotic signaling pathways. Regul. Pept 2006, 137, 20–26. [Google Scholar]
- Racz, B; Gallyas, F, Jr; Kiss, P; Tamas, A; Lubics, A; Lengvari, I; Roth, E; Toth, G; Hegyi, O; Verzar, Zs; Fabricsek, Cs; Reglodi, D. Effects of pituitary adenylate cyclase activating polypeptide (PACAP) on the PKA-Bad-14-3-3 signaling pathway in glutamate-induced retinal injury in neonatal rats. Neurotox. Res 2007, 12, 95–104. [Google Scholar]
- Atlasz, T; Szabadfi, K; Kiss, P; Tamas, A; Toth, G; Reglodi, D; Gabriel, R. Evaluation of the protective effects of PACAP with cell-specific markers in ischemia-induced retinal degeneration. Brain Res Bull 2009, in press. [Google Scholar]
- Virag, L; Szabo, C. The therapeutic potential of poly(ADPribose) polymerase inhibitors. Pharmacol. Rev 2002, 54, 375–429. [Google Scholar]
- Pacher, P; Szabo, C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am. J. Pathol 2008, 173, 2–13. [Google Scholar]
- Halmosi, R; Berente, Z; Osz, E; Toth, K; Literati-Nagy, P; Sumegi, B. Effect of poly(ADP-ribose) polymerase inhibitors on the ischemia-reperfusion-induced oxidative cell damage and mitochondrial metabolism in Langendorff heart perfusion system. Mol. Pharmacol 2001, 59, 1497–1505. [Google Scholar]
- Hong, SJ; Dawson, TM; Dawson, VL. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol. Sci 2004, 25, 259–264. [Google Scholar]
- Yu, SW; Wang, H; Poitras, MF; Coombs, C; Bowers, WJ; Federoff, HJ; Poirier, GG; Dawson, TM; Dawson, VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 2002, 297, 259–263. [Google Scholar]
- Xu, Y; Huang, S; Liu, ZG; Han, J. Poly(ADP-ribose) polymerase-1 signaling to mitochondria in necrotic cell death requires RIP1/TRAF2-mediated JNK1 activation. J. Biol. Chem 2006, 281, 8788–8795. [Google Scholar]
- Veres, B; Gallyas, F, Jr; Varbiro, G; Berente, Z; Osz, E; Szekeres, G; Szabo, C; Sumegi, B. Decrease of the inflammatory response and induction of the Akt/protein kinase B pathway by poly-(ADP-ribose) polymerase 1 inhibitor in endotoxin-induced septic shock. Biochem. Pharmacol 2003, 65, 1373–1382. [Google Scholar]
- Weise, J; Isenmann, S; Bahr, M. Increased expression and activation of poly(ADP-ribose) polymerase (PARP) contribute to retinal ganglion cell death following rat optic nerve transection. Cell Death Differ 2001, 8, 801–807. [Google Scholar]
- Paquet-Durand, F; Silva, J; Talukdar, T; Johnson, LE; Azadi, S; van Veen, T; Ueffing, M; Hauck, SM; Ekstrom, PA. Excessive activation of poly-(ADP-ribose) polymerase contributes to inherited photoreceptor degeneration in the retinal degeneration 1 mouse. Neurobiol. Dis 2007, 27, 10311–10319. [Google Scholar]
- Li, GY; Osborne, NN. Oxidative-induced apoptosis to an immortalized ganglion cell line is caspase independent but involves the activation of poly (ADP-ribose) polymerase and apoptosis-inducing factor. Brain Res 2008, 1188, 35–43. [Google Scholar]
- Goebel, DJ; Winkler, BS. Blockade of PARP activity attenuates poly(ADP-ribosyl)ation but offers only partial neuroprotection against NMDA-induced cell death in the rat retina. J. Neurochem 2006, 98, 1732–1745. [Google Scholar]
- Uehara, N; Miki, K; Tsukamoto, R; Matsuoka, Y; Tsubura, A. Nicotinamide blocks N-methyl-N-nitrosourea-induced photoreceptor cell apoptosis in rats through poly (ADP-ribose) polymerase activity and Jun N-terminal kinase/activator protein-1 pathway inhibition. Exp. Eye Res 2006, 82, 488–495. [Google Scholar]
- Ferrer, I; Planas, AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J. Neuropathol. Exp. Neurol 2003, 62, 329–339. [Google Scholar]
- Meli, E; Pangallo, M; Baronti, R; Chiarugi, A; Cozzi, A; Pellegrini-Giampietro, DE; Moroni, F. Poly(ADP-ribose) polymerase as a key player in excitotoxicity and post-ischemic brain damage. Toxicol. Lett 2003, 139, 153–162. [Google Scholar]
- Ikeda, Y; Hokamura, K; Kawai, T; Ishiyama, J; Ishikawa, K; Anraku, T; Uno, T; Umemura, K. Neuroprotective effects of KCL-440, a new poly(ADP-ribose) polymerase inhibitor, in the rat middle cerebral artery occlusion model. Brain Res 2005, 1060, 73–80. [Google Scholar]
- Cozzi, A; Cipriani, G; Fossati, S; Faraco, G; Formentini, L; Min, W; Cortes, U; Wang, ZQ; Moroni, F; Chiarugi, A. Poly(ADPribose) accumulation and enhancement of postischemic brain damage in 110-kDa poly(ADP-ribose) glycohydrolase null mice. J. Cereb. Blood Flow Metab 2006, 26, 684–695. [Google Scholar]


| Substance | Main effects | References |
|---|---|---|
| Antioxidants (e.g., Vitamin E, lutein, flavonoids) | ↓ oxidative damage ↓ caspase3 ↑ glutathione ↓ nitrotyrosine ↓ nuclear PAR ↓ loss of ATP | [23] [24] [25] |
| Ischemic preconditioning | protein kinase C activation ATP-sensitive K+ channel opening ↑ ferritin level adenosine A1 receptor stimulation STAT-3 activation ↑ HSP27 | [26] [27] [28] [29] [30] |
| Ischemic postconditioning | ↓ glutamate | [31] |
| Adenosine | vasodilation ↓ neuronal activity ↑ glycogenolysis | [32] |
| Growth factors (IGFII, NGF, BDNF, VEGF) | ↑ phosphate activated glutaminase (PAG) ↓ ammonia ↑ blood flow to the retina ↑ Bcl-2 ↓ Bax | [33] [34] [35] |
| Erythropoietin | ↓ apoptosis ↑ ischemic preconditioning | [36] [37] [38] |
| Statins | ↓ HSP27 | [39] |
| Estradiol | ↓ glutamate | [40] |
| Cannabinoids | ↓ peroxynitrite | [41] |
| Morphine | ↑ ischemic preconditioning | [42,43] |
| L-carnitine | ↓ oxidative stress | [44] |
| Glutamate receptor antagonists | ↓ glutamate excitotoxicity | [45] |
| Adrenergic receptor blockers | ↓ influx of sodium and calcium | [46] |
| Alpha2 adrenergic agonist (brimonidine) | ↓ glutamate and aspartate | [47] |
| Ca2+, K+, Na+ channel blockers | ↓ influx of sodium and calcium ↑ ischemic preconditioning ↓ c-jun, p-JNK | [48,49] |
| Hypothermia | ↓ energy demand | [50] |
| Hyperglycaemia | ↑ HSP-27 | [51] |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Szabadfi, K.; Mester, L.; Reglodi, D.; Kiss, P.; Babai, N.; Racz, B.; Kovacs, K.; Szabo, A.; Tamas, A.; Gabriel, R.; et al. Novel Neuroprotective Strategies in Ischemic Retinal Lesions. Int. J. Mol. Sci. 2010, 11, 544-561. https://doi.org/10.3390/ijms11020544
Szabadfi K, Mester L, Reglodi D, Kiss P, Babai N, Racz B, Kovacs K, Szabo A, Tamas A, Gabriel R, et al. Novel Neuroprotective Strategies in Ischemic Retinal Lesions. International Journal of Molecular Sciences. 2010; 11(2):544-561. https://doi.org/10.3390/ijms11020544
Chicago/Turabian StyleSzabadfi, Krisztina, Laszlo Mester, Dora Reglodi, Peter Kiss, Norbert Babai, Boglarka Racz, Krisztina Kovacs, Aliz Szabo, Andrea Tamas, Robert Gabriel, and et al. 2010. "Novel Neuroprotective Strategies in Ischemic Retinal Lesions" International Journal of Molecular Sciences 11, no. 2: 544-561. https://doi.org/10.3390/ijms11020544