Synergistic Efficacy of Plaque Control with Intralesional Triamcinolone Acetonide Injection on Erosive Non-Gingival Oral Lichen Planus: A Randomized Controlled Clinical Trial
Abstract
:1. Introduction
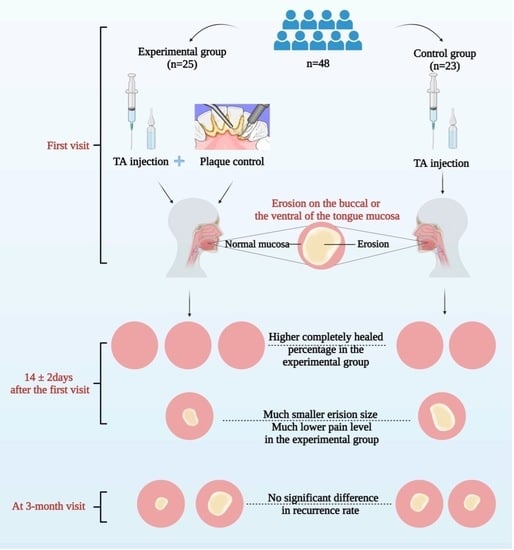
2. Materials and Methods
2.1. Participants
2.2. Randomization
2.3. Study Interventions
2.4. Clinical Assessment
2.5. Adverse Reactions
2.6. Blind Evaluation
2.7. Follow-Up Assessment
2.8. Statistical Analysis
3. Results
3.1. Participants
3.2. Baseline
3.3. Efficacy Analysis
3.4. Improvement of Periodontal Status
3.5. Recurrence Analysis
3.6. Safety Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Moles, M.; Warnakulasuriya, S.; González-Ruiz, I.; González-Ruiz, L.; Ayén, Á.; Lenouvel, D.; Ruiz-Ávila, I.; Ramos-García, P. Worldwide prevalence of oral lichen planus: A systematic review and meta-analysis. Oral Dis. 2021, 27, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, G.; Zeng, H.; Xiong, C.R.; Lin, M.; Zhou, H.M. A randomized double-blind, positive-control trial of topical thalidomide in erosive oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 110, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Dillenburg, C.S.; Martins, M.A.; Munerato, M.C.; Marques, M.M.; Carrard, V.C.; Sant’Ana Filho, M.; Castilho, R.M.; Martins, M.D. Efficacy of laser phototherapy in comparison to topical clobetasol for the treatment of oral lichen planus: A randomized controlled trial. J. Biomed. Opt. 2014, 19, 068002. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Li, Q.; Lin, M.; Li, X.; Meng, W.; Wu, Y.; Zeng, X.; Zhou, H.; Zhou, G. The efficacy of topical intralesional BCG-PSN injection in the treatment of erosive oral lichen planus: A randomized controlled trial. J. Oral Pathol. Med. 2009, 38, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Aghbari, S.M.H.; Abushouk, A.I.; Attia, A.; Elmaraezy, A.; Menshawy, A.; Ahmed, M.S.; Elsaadany, B.A.; Ahmed, E.M. Malignant transformation of oral lichen planus and oral lichenoid lesions: A meta-analysis of 20095 patient data. Oral Oncol. 2017, 68, 92–102. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.; Ruiz-Ávila, I.; González-Ruiz, L.; Ayén, Á.; Gil-Montoya, J.A.; Ramos-García, P. Malignant transformation risk of oral lichen planus: A systematic review and comprehensive meta-analysis. Oral Oncol. 2019, 96, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.; Troiano, G.; Cordaro, M.; Corsalini, M.; Gioco, G.; Lo Muzio, L.; Pignatelli, P.; Lajolo, C. Rate of malignant transformation of oral lichen planus: A systematic review. Oral Dis. 2019, 25, 693–709. [Google Scholar] [CrossRef]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardiñas López, S.; Shanti, R.M. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef]
- Ramos-García, P.; González-Moles, M.; Warnakulasuriya, S. Oral cancer development in lichen planus and related conditions-3.0 evidence level: A systematic review of systematic reviews. Oral Dis. 2021, 27, 1919–1935. [Google Scholar] [CrossRef]
- Arduino, P.G.; Campolongo, M.G.; Sciannameo, V.; Conrotto, D.; Gambino, A.; Cabras, M.; Ricceri, F.; Carossa, S.; Broccoletti, R.; Carbone, M. Randomized, placebo-controlled, double-blind trial of clobetasol propionate 0.05% in the treatment of oral lichen planus. Oral Dis. 2018, 24, 772–777. [Google Scholar] [CrossRef]
- Lodi, G.; Manfredi, M.; Mercadante, V.; Murphy, R.; Carrozzo, M. Interventions for treating oral lichen planus: Corticosteroid therapies. Cochrane Database Syst. Rev. 2020, 2, Cd001168. [Google Scholar] [CrossRef] [PubMed]
- Ioannides, D.; Vakirlis, E.; Kemeny, L.; Marinovic, B.; Massone, C.; Murphy, R.; Nast, A.; Ronnevig, J.; Ruzicka, T.; Cooper, S.M.; et al. European S1 guidelines on the management of lichen planus: A cooperation of the European Dermatology Forum with the European Academy of Dermatology and Venereology. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Solimani, F.; Forchhammer, S.; Schloegl, A.; Ghoreschi, K.; Meier, K. Lichen planus—A clinical guide. J. Dtsch Dermatol. Ges 2021, 19, 864–882. [Google Scholar] [CrossRef]
- Łukaszewska-Kuska, M.; Ślebioda, Z.; Dorocka-Bobkowska, B. The effectiveness of topical forms of dexamethasone in the treatment of oral lichen planus- A systematic review. Oral Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, S.; Azari-Marhabi, S.; Mojahedi, S.M.; Namdari, M.; Rankohi, Z.E.; Jafari, S. Comparing clinical effects of photodynamic therapy as a novel method with topical corticosteroid for treatment of Oral Lichen Planus. Photodiagnosis Photodyn Ther. 2017, 20, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moles, M.A.; Bravo, M.; Gonzalez-Ruiz, L.; Ramos, P.; Gil-Montoya, J.A. Outcomes of oral lichen planus and oral lichenoid lesions treated with topical corticosteroid. Oral Dis. 2018, 24, 573–579. [Google Scholar] [CrossRef]
- Conrotto, D.; Carbone, M.; Carrozzo, M.; Arduino, P.; Broccoletti, R.; Pentenero, M.; Gandolfo, S. Ciclosporin vs. clobetasol in the topical management of atrophic and erosive oral lichen planus: A double-blind, randomized controlled trial. Br. J. Dermatol. 2006, 154, 139–145. [Google Scholar] [CrossRef]
- López-Jornet, P.; Camacho-Alonso, F. Application of a motivation-behavioral skills protocol in gingival lichen planus: A short-term study. J. Periondotol. 2010, 81, 1449–1454. [Google Scholar] [CrossRef]
- Stone, S.J.; McCracken, G.I.; Heasman, P.A.; Staines, K.S.; Pennington, M. Cost-effectiveness of personalized plaque control for managing the gingival manifestations of oral lichen planus: A randomized controlled study. J. Clin. Periondotol. 2013, 40, 859–867. [Google Scholar] [CrossRef] [Green Version]
- Holmstrup, P.; Schiøtz, A.W.; Westergaard, J. Effect of dental plaque control on gingival lichen planus. Oral Surg. Oral Med. Oral Pathol. 1990, 69, 585–590. [Google Scholar] [CrossRef]
- Salgado, D.S.; Jeremias, F.; Capela, M.V.; Onofre, M.A.; Massucato, E.M.; Orrico, S.R. Plaque control improves the painful symptoms of oral lichen planus gingival lesions. A short-term study. J. Oral Pathol. Med. 2013, 42, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Scattarella, A.; Petruzzi, M.; Ballini, A.; Grassi, F.; Nardi, G. Oral lichen planus and dental hygiene: A case report. Int. J. Dent. Hyg. 2011, 9, 163–166. [Google Scholar] [CrossRef] [PubMed]
- van der Meij, E.H.; van der Waal, I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J. Oral Pathol. Med. 2003, 32, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Turesky, S.; Gilmore, N.D.; Glickman, I. Reduced plaque formation by the chloromethyl analogue of victamine C. J. Periondotol. 1970, 41, 41–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutress, T.W.; Ainamo, J.; Sardo-Infirri, J. The community periodontal index of treatment needs (CPITN) procedure for population groups and individuals. Int. Dent. J. 1987, 37, 222–233. [Google Scholar] [PubMed]
- Dentkos, T.R.; Berzins, D.W. Evaluation of cutting efficiency of orthograde ultrasonic tips by using a nonstatic model. J. Endod. 2008, 34, 863–865. [Google Scholar] [CrossRef]
- Park, H.K.; Hurwitz, S.; Woo, S.B. Oral lichen planus: REU scoring system correlates with pain. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 75–82. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Pshycol. 2013, 4, 863. [Google Scholar] [CrossRef] [Green Version]
- Bakker, A.; Cai, J.; English, L.; Kaiser, G.; Mesa, V.; Van Dooren, W. Beyond small, medium, or large: Points of consideration when interpreting effect sizes. Educ. Stud. Math. 2019, 102, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.C.; Shin, S.Y.; Kim, S.W.; Eun, Y.G. Intralesional injection versus mouth rinse of triamcinolone acetonide in oral lichen planus: A randomized controlled study. Otolaryngol. Head Neck Surg. 2013, 148, 443–449. [Google Scholar] [CrossRef]
- Xia, J.; Li, C.; Hong, Y.; Yang, L.; Huang, Y.; Cheng, B. Short-term clinical evaluation of intralesional triamcinolone acetonide injection for ulcerative oral lichen planus. J. Oral Pathol. Med. 2006, 35, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.J.; Heasman, P.A.; Staines, K.S.; McCracken, G.I. The impact of structured plaque control for patients with gingival manifestations of oral lichen planus: A randomized controlled study. J. Clin. Periondotol. 2015, 42, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Guiglia, R.; Di Liberto, C.; Pizzo, G.; Picone, L.; Lo Muzio, L.; Gallo, P.D.; Campisi, G.; D’Angelo, M. A combined treatment regimen for desquamative gingivitis in patients with oral lichen planus. J. Oral Pathol. Med. 2007, 36, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Ertugrul, A.S.; Dursun, R.; Dundar, N.; Avunduk, M.C.; Hakki, S.S. MMP-1, MMP-9, and TIMP-1 levels in oral lichen planus patients with gingivitis or periodontitis. Arch. Oral Biol. 2013, 58, 843–852. [Google Scholar] [CrossRef]
- Romano, F.; Arduino, P.G.; Maggiora, M.; Curmei, E.; Manavella, V.; Broccoletti, R.; Aimetti, M. Effect of a structured plaque control on MMP-1 and MMP-9 crevicular levels in patients with desquamative gingivitis associated with oral lichen planus. Clin. Oral Investig. 2019, 23, 2651–2658. [Google Scholar] [CrossRef]
- Werneck, J.T.; Costa Tde, O.; Stibich, C.A.; Leite, C.A.; Dias, E.P.; Silva Junior, A. Oral lichen planus: Study of 21 cases. An. Bras. Dermatol. 2015, 90, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Krogh, P.; Holmstrup, P.; Thorn, J.J.; Vedtofte, P.; Pindborg, J.J. Yeast species and biotypes associated with oral leukoplakia and lichen planus. Oral Surg. Oral Med. Oral Pathol. 1987, 63, 48–54. [Google Scholar] [CrossRef]
- Reynaud, A.H.; Nygaard-Østby, B.; Bøygard, G.K.; Eribe, E.R.; Olsen, I.; Gjermo, P. Yeasts in periodontal pockets. J. Clin. Periondotol. 2001, 28, 860–864. [Google Scholar] [CrossRef]
- Masetti, P.; Sanitá, P.V.; Jorge, J.H. Dynamics and metabolic profile of oral keratinocytes (NOK-si) and Candida albicans after interaction in co-culture. Biofouling 2021, 37, 572–589. [Google Scholar] [CrossRef]
- Feller, L.; Khammissa, R.A.; Chandran, R.; Altini, M.; Lemmer, J. Oral candidosis in relation to oral immunity. J. Oral Pathol. Med. 2014, 43, 563–569. [Google Scholar] [CrossRef]
- Pärnänen, P.; Meurman, J.H.; Samaranayake, L.; Virtanen, I. Human oral keratinocyte E-cadherin degradation by Candida albicans and Candida glabrata. J. Oral Pathol. Med. 2010, 39, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.V.; Barbosa, G.M.; Higashi, D.; di Micheli, G.; Rodrigues, P.H.; Simionato, M.R.L. Quantitative detection of Staphylococcus aureus, Enterococcus faecalis and Pseudomonas aeruginosa in human oral epithelial cells from subjects with periodontitis and periodontal health. J. Med. Microbiol. 2013, 62, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Dias, K.C.; Barbugli, P.A.; Vergani, C.E. Insights into the activation of oral keratinocyte cell death by Candida albicans and Staphylococcus aureus biofilms. Biofouling 2021, 37, 975–983. [Google Scholar] [CrossRef] [PubMed]


| Experimental Group (n = 25) | Control Group (n = 23) | p Value | |
|---|---|---|---|
| Age, year (mean ± SD) | 46.5 ± 9.9 | 49 ± 11.4 | 0.42 |
| Sex, number (%) | 0.68 | ||
| Male | 9 (36.0%) | 7 (30.4%) | |
| Female | 16 (64.0%) | 16 (69.6%) | |
| Position, number (%) | 1 | ||
| Buccal | 23 (92.0%) | 21 (91.3%) | |
| Ventral of the tongue | 2 (8.0%) | 2 (8.7%) | |
| OLP duration, month (mean ± SD) | 26.2 ± 15.8 | 31.1 ± 18.9 | 0.33 |
| Group | Completely Healed (%) | Not Completely Healed (%) | Total |
|---|---|---|---|
| E | 23 (95.8%) | 1 (4.2%) | 24 |
| C | 16 (69.6%) | 7 (30.4%) | 23 |
| Total | 39 | 8 | 47 |
| Group | Number | Mean | SD | bp Value | c Cohen’s d (95% CI) | |
|---|---|---|---|---|---|---|
| Erosion size (mm2) | ||||||
| First visit | E | 25 | 22.3 | 20.4 | 0.40 | |
| C | 23 | 16.0 | 12.4 | |||
| a Reduced erosion area at day 14 ± 2 | E | 24 | 19.1 | 12.8 | 0.029 * | 0.68 (0.08, 1.28) |
| C | 23 | 10.3 | 13.2 | |||
| NRS score | ||||||
| First visit | E | 25 | 4.3 | 1.8 | 0.29 | |
| C | 23 | 4.9 | 1.7 | |||
| a Reduced NRS at day 14 ± 2 | E | 24 | 3.9 | 1.7 | 0.036 * | 0.63 (0.03, 1.23) |
| C | 23 | 2.6 | 2.4 | |||
| PI | ||||||
| First visit | E | 25 | 2.6 | 1.3 | 0.95 | |
| C | 23 | 2.5 | 0.9 | |||
| a Reduced PI at day 14 ± 2 | E | 24 | 1.7 | 0.9 | <0.001 *** | 1.29 (0.65, 1.93) |
| C | 23 | 0.6 | 0.8 | |||
| CPI | ||||||
| First visit | E | 25 | 2.0 | 0.8 | 0.10 | |
| C | 23 | 2.4 | 0.7 | |||
| a Reduced CPI at day 14 ± 2 | E | 24 | 1.6 | 0.9 | <0.001 *** | 1.36 (0.71, 2.01) |
| C | 23 | 0.5 | 0.7 |
| Group | Not Recurred (%) | b Recurred (%) | Total |
|---|---|---|---|
| E | 15 (68.2%) | 7 (31.8%) | 22 |
| C | 10 (62.5%) | 6 (37.5%) | 16 |
| Total | 26 | 12 | 38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Lin, D.; Deng, S.; Wang, S.; Guo, Y.; Yang, J.; Shi, X.; Zhou, H. Synergistic Efficacy of Plaque Control with Intralesional Triamcinolone Acetonide Injection on Erosive Non-Gingival Oral Lichen Planus: A Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 13787. https://doi.org/10.3390/ijerph192113787
Zhao W, Lin D, Deng S, Wang S, Guo Y, Yang J, Shi X, Zhou H. Synergistic Efficacy of Plaque Control with Intralesional Triamcinolone Acetonide Injection on Erosive Non-Gingival Oral Lichen Planus: A Randomized Controlled Clinical Trial. International Journal of Environmental Research and Public Health. 2022; 19(21):13787. https://doi.org/10.3390/ijerph192113787
Chicago/Turabian StyleZhao, Wei, Duanxian Lin, Shuzhi Deng, Shimeng Wang, Yiqing Guo, Jin Yang, Xueke Shi, and Hongmei Zhou. 2022. "Synergistic Efficacy of Plaque Control with Intralesional Triamcinolone Acetonide Injection on Erosive Non-Gingival Oral Lichen Planus: A Randomized Controlled Clinical Trial" International Journal of Environmental Research and Public Health 19, no. 21: 13787. https://doi.org/10.3390/ijerph192113787