A Comprehensive Review on Multifaceted Mechanisms Involved in the Development of Breast Cancer Following Adverse Childhood Experiences (ACEs)
Abstract
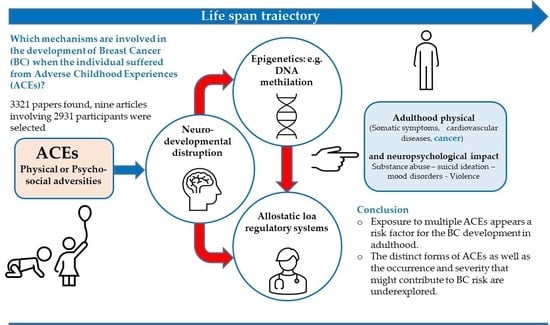
:1. Introduction
2. Materials and Methods
2.1. Literature Search Methodology
2.2. Eligibility Criteria and Study Selection
3. Results
3.1. Types of ACEs
3.2. Methods Used to Manage the Occurrence of ACEs
3.3. Stress Vulnerability and Its Clinical Expressions
3.4. Inflammation Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahri, N.; Fathi Najafi, T.; Homaei Shandiz, F.H.; Tohidinik, H.R.; Khajavi, A. The relation between stressful life events and breast cancer: A systematic review and meta-analysis of cohort studies. Breast Cancer Res. Treat. 2019, 176, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Petticrew, M.; Fraser, J.; Regan, M.F. Adverse life-events and risk of breast cancer: A meta-analysis. Br. J. Health Psychol. 1999, 4, 1–17. [Google Scholar] [CrossRef]
- Chen, M.A.; LeRoy, A.S.; Majd, M.; Chen, J.Y.; Brown, R.L.; Christian, L.M.; Fagundes, C.P. Immune and epigenetic pathways linking childhood adversity and health across the lifespan. Front. Psychol. 2021, 12, 788351. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.P. The key role of epigenetics in human disease prevention and mitigation. N. Engl. J. Med. 2018, 378, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Lehrner, A. Intergenerational transmission of trauma effects: Putative role of epigenetic mechanisms. World Psychiatry 2018, 17, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Hanssen, L.M.; Schutte, N.S.; Malouff, J.M.; Epel, E.S. The relationship between childhood psychosocial stressor level and telomere length: A meta-analysis. Health Psychol. Res. 2017, 5, 6378. [Google Scholar] [CrossRef] [Green Version]
- Tyrka, A.R.; Parade, S.H.; Price, L.H.; Kao, H.-T.; Porton, B.; Philip, N.S.; Welch, E.S.; Carpenter, L.L. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biol. Psychiatry 2016, 79, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Lindqvist, D.; Epel, E.S.; Mellon, S.H.; Penninx, B.W.; Révész, D.; Verhoeven, J.E.; Reus, V.I.; Lin, J.; Mahan, L.; Hough, C.M.; et al. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci. Biobehav. Rev. 2015, 55, 333–364. [Google Scholar] [CrossRef] [Green Version]
- Rentscher, K.E.; Carroll, J.E.; Mitchell, C. Psychosocial stressors, and telomere length: A current review of the science. Annu. Rev. Public Health 2020, 41, 223–245. [Google Scholar] [CrossRef] [Green Version]
- Norman, R.E.; De Byambaa, R.; Butchart, A.; Scott, J.; Vos, T. The long-term health consequences of child physical abuse emotional abuse and neglect: A systematic review and meta-analysis. PLoS Med. 2012, 9, e1001349. [Google Scholar] [CrossRef]
- Alisic, E.; Zalta, A.K.; Van Wesel, F.; Larsen, S.E.; Hafstad, G.S.; Hassanpour, K.; Smid, G.E. Rates of post-traumatic stress disorder in trauma-exposed children and adolescents: Meta-analysis. Br. J. Psychiatry 2014, 204, 335–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, D.J.; Fernandez Poole, S.; White, M.; Lyn, R.; Flores, D.A.; Haile, H.G.; Williams, D.R. The role of stress in breast cancer incidence: Risk factors, interventions, and directions for the future. Int. J. Environ. Res. Public Health. 2021, 18, 1871. [Google Scholar] [CrossRef] [PubMed]
- Boynton-Jarrett, R.; Fargnoli, J.; Suglia, S.F.; Zuckerman, B.; Wright, R.J. Association between maternal intimate partner violence and incident obesity in preschool-aged children: Results from the fragile families and child well-being study. Arch. Pediat. Adol. Med. 2010, 164, 540–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, P.M.; Mustillo, S.A.; Ferraro, K.F. Does childhood misfortune raise the risk of acute myocardial infarction in adulthood? Soc. Sci. Med. 2014, 104, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmiston, E.E.; Wang, F.; Mazure, C.M. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch. Pediat. Adol. Med. 2011, 165, 1069–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pino, O. Ricucire i Ricordi. La Memoria i suoi Disturbi Evidenze di Efficacia dei Trattamenti Riabilitativi; Ed. Mondadori Education: Milano, Italy, 2007. [Google Scholar]
- Wierenga, L.M.; Langen, M.; Oranje, B.; Durston, S. Unique developmental trajectories of cortical thickness and surface area. NeuroImage 2014, 87, 120–126. [Google Scholar] [CrossRef]
- Bruce, S.; Buchholz, K.; Brown, W.; Yan, L.; Durbin, A.; Sheline, Y. Altered emotional interference processing in the amygdala and insula in women with Post-Traumatic Stress Disorder. NeuroImage Clin. 2012, 2, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Shou, H.; Yang, Z.; Satterthwaite, T.; Cook, P.; Bruce, S.; Shinohara, R.; Rosenberg, B.; Sheline, Y. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. NeuroImage Clin. 2017, 14, 464–470. [Google Scholar] [CrossRef]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The adverse childhood experiences (ACE) study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Goldsmith, R.E.; Jandorf, L.; Valdimarsdottir, H.; Amend, K.L.; Stoudt, B.G.; Rini, C.; Hershman, D.; Neugut, A.; Reilly, J.; Tartter, P.; et al. Traumatic stress symptoms and breast cancer: The role of childhood abuse. Child Abuse Negl. 2010, 34, 465–470. [Google Scholar] [CrossRef]
- Salmon, P.; Hill, J.; Krespi, R.; Clark, L.; Fisher, J.; Holcombe, C. The role of child abuse and age in vulnerability to emotional problems after surgery for breast cancer. Eur. J. Cancer 2006, 42, 2517–2523. [Google Scholar] [CrossRef] [PubMed]
- Bandinelli, L.P.; Levandowski, M.L.; Grassi-Oliveira, R. The childhood maltreatment influences on breast cancer patients: A second wave hit model hypothesis for distinct biological and behavioral response. Med. Hypotheses 2017, 108, 86–93. [Google Scholar] [CrossRef]
- Warner, E.; Hargreaves, M.K.; Mouton, C.P.; Tamimi, R.M.; Signorello, L.B. Adverse childhood experiences and breast cancer risk factors in black and white women. In Proceedings of the Eighth AACR Conference on The Science of Health Disparities in Racial/Ethnic Minorities and the Medically Underserved, Atlanta, GA, USA, 13–16 November 2015. [Google Scholar]
- Nelson, C.A.; Bhutta, Z.A.; Burke Harris, N.; Danese, A.; Samara, M. Adversity in childhood is linked to mental and physical health throughout life. BMJ 2020, 371, m3048. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.L. Screening for Adverse Childhood Experiences (ACEs) in primary care: A cautionary note. JAMA 2020, 323, 2379–2380. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, R.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane Training. 2020. Available online: https://www.training.cochrane.org/handbook (accessed on 30 June 2022).
- Wahbeh, H.; Heinz, B.; Fry, N.; Wojakowski, M. Exploring lifetime experiences of people with breast cancer: A cross-sectional study. J. Oncol. Res. Treat. 2021, 6, 160. [Google Scholar] [CrossRef]
- Crosswell, A.D.; Bower, J.E.; Ganz, P.A. Childhood adversity and inflammation in breast cancer survivors. Psychosom. Med. 2014, 76, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Fagundes, C.P.; Glaser, R.; Malarkey, W.B.; Kiecolt-Glaser, J.K. Childhood adversity and herpesvirus latency in breast cancer survivors. Health Psychol. 2013, 32, 337–344. [Google Scholar] [CrossRef]
- Kamen, C.; Scheiber, C.; Janelsins, M.; Jo, B.; Shen, H.; Palesh, O. Effects of childhood trauma exposure and cortisol levels on cognitive functioning among breast cancer survivors. Child Abuse Negl. 2017, 72, 163–171. [Google Scholar] [CrossRef] [PubMed]
- McFarland, D.C.; Andreotti, C.; Harris, K.; Mandeli, J.; Tiersten, A.; Holland, J. Early childhood adversity and its associations with anxiety depression and distress in women with breast cancer. Psychosomatics 2016, 57, 174–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, T.J.; Felger, J.C.; Lee, A.; Mister, D.; Miller, A.H.; Torres, M.A. Association of childhood trauma with fatigue depression stress and inflammation in breast cancer patients undergoing radiotherapy. Psychooncology 2016, 25, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witek-Janusek, L.; Tell, D.; Albuquerque, K.; Mathews, H.L. Childhood adversity increases vulnerability for behavioral symptoms and immune dysregulation in women with breast cancer. Brain Behav. Immun. 2013, 30, S149–S162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bower, J.E.; Kuhlman, K.R.; Ganz, P.A.; Irwin, M.R.; Crespi, C.M.; Cole, S.W. Childhood maltreatment and monocyte gene expression among women with breast cancer. Brain Behav. Immun. 2020, 88, 396–402. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. BDI-II: Beck Depression Inventory 2; Harcourt Brace Inc.: Boston, MA, USA, 1996. [Google Scholar]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Berstein, D.P. Childhood Trauma Questionnaire: A Retrospective Self-Report; The Psychological Corporation: San Antonio, TX, USA, 1998. [Google Scholar]
- Smets, E.M.; Garssen, B.; Cull, A.; de Haes, J.C. Application of the multidimensional fatigue inventory (MFI-20) in cancer patients receiving radiotherapy. Br. J. Cancer 1996, 73, 241–245. [Google Scholar] [CrossRef] [Green Version]
- Arjadi, R.; Nauta, M.H.; Utoyo, D.B.; Bockting, C. The Inventory of Depressive Symptomatology Self Report (IDS-SR): Psychometric properties of the Indonesian version. PLoS ONE 2017, 12, e018700. [Google Scholar] [CrossRef] [Green Version]
- Hann, D.; Winter, K.; Jacobsen, P. Measurement of depressive symptoms in cancer patients: Evaluation of the Center for Epidemiological Studies Depression Scale (CES-D). J. Psychosom. Res. 1999, 46, 437–443. [Google Scholar] [CrossRef]
- Pickard-Holley, S. Fatigue in cancer patients A descriptive study. Cancer Nurs. 1991, 14, 13–19. [Google Scholar] [CrossRef]
- Stone, P.; Richards, M.; Hardy, J. Fatigue in patients with cancer. Eu. J. Cancer 1998, 34, 1670–1676. [Google Scholar] [CrossRef]
- Ferrans, C.E. Development of a quality of life index for patients with cancer. Oncol. Nurs. Forum 1990, 17, 15–19. [Google Scholar] [PubMed]
- Briere, J. Psychological Assessment of Adult Posttraumatic States; American Psychological Association: Washington, DC, USA, 1997. [Google Scholar]
- Wagner, L.; Sweet, J.; Butt, Z.; Lai, J.; Cella, D. Measuring patient self-reported cognitive function: Development of the functional assessment of cancer therapy-cognitive function instrument. J. Support. Oncol. 2009, 7, 32–39. [Google Scholar]
- Savard, M.H.; Savard, J.; Simard, S.; Ivers, H. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology 2005, 14, 429–441. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Sydeman, S.J.; Owen, A.E.; Marsh, B.J. Measuring anxiety and anger with the state-Trait anxiety inventory (STAI) and the state-Trait anger expression inventory (STAXI). In The Use of Psychological Testing for Treatment Planning and Outcomes Assessment, 2nd ed.; Maruish, M.E., Ed.; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 1999; pp. 993–1021. [Google Scholar]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.I.; Monk, T.H.; Berman, S.B.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psych. Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Distress Management v22019 Referenced with Permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines¯) for Distress Management V22019 © National Comprehensive Cancer Network Inc. 2019. Available online: http://www.nccn.org/professionals/physician_gls/distress.pdf (accessed on 23 February 2020).
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, M.; Wilner, N.; Alvarez, W. Impact of event scale: A measure of subjective stress. Psychosom. Med. 1979, 41, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Bremner, J.D. Long-term effects of childhood abuse on brain and neurobiology. Child Adolesc. Psychiatr. Clin. 2003, 12, 271–292. [Google Scholar] [CrossRef]
- Briere, J. Treating adult survivors of severe childhood abuse and neglect: Further development of an integrative model. In The APSAC Handbook on Child Maltreatment, 2nd ed.; Meyers, J.E.B., Berliner, L., Briere, J., Hendrix, C.T., Reid, T., Jenny, C., Eds.; Sage: Thousand Oaks, CA, USA, 2002; pp. 175–203. [Google Scholar]
- De Bellis, M.D. Developmental traumatology: The psychobiological development of maltreated children and its implications for research treatment and policy. Dev. Psychopath. 2001, 13, 539–564. [Google Scholar] [CrossRef]
- Schore, A.N. The effects of early relational trauma on right brain development affect regulation and infant mental health. Infant Ment. Health J. 2001, 22, 201–269. [Google Scholar] [CrossRef]
- Bernstein, D.P.; Stein, J.A.; Newcomb, M.D.; Walker, E.; Pogge, D.; Ahluvalia, T.; Stokes, J.; Handelsman, L.; Medrano, M.; Desmond, D.; et al. Development and validation of a brief screening version of the Child Trauma Questionnaire. Child Abuse Neglect. 2003, 27, 169–190. [Google Scholar] [CrossRef]
- Levine, M.E.; Cole, S.W.; Weir, D.R.; Crimmins, E.M. Childhood and later life stressors and increased inflammatory gene expression at older ages. Soc. Sci. Med. 2015, 130, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, S.-M.; Leng, L.-L.; Xie, Q.W.; Chan, J.S.M.; Chan, C.H.Y.; So, K.F.; Li, A.; Po, K.K.T.; Yuen, L.P.; Ku, K.-S.; et al. Trust as a mediator in the relationship between childhood sexual abuse and IL-6 level in adulthood. PLoS ONE 2020, 15, e0232932. [Google Scholar] [CrossRef]
- Carpenter, L.L.; Shattuck, T.T.; Tyrka, A.R.; Geracioti, T.D.; Price, L.H. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology 2011, 214, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.J.; Arseneault, L.; Caspi, A.; Fisher, H.L.; Matthews, T.; Moffitt, T.E.; Odgers, C.L.; Stahl, D.; Teng, J.Y.; Danese, A. The epidemiology of trauma and post-traumatic stress disorder in a representative cohort of young people in England and Wales. Lancet Psychiatry 2019, 6, 247–256. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, K.A.; Conron, K.J.; Koenen, K.C.; Gilman, S.E. Childhood adversity adult stressful life events and risk of past-year psychiatric disorder: A test of the stress sensitization hypothesis in a population-based sample of adults. Psychol. Med. 2010, 40, 1647–1658. [Google Scholar] [CrossRef] [Green Version]
- Teicher, M.H.; Samson, J.A. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatry 2013, 170, 1114–1133. [Google Scholar] [CrossRef]
- Gust, D.A.; Gordon, T.P.; Hambright, M.K.; Wilson, M.E. Relationship between social factors and pituitary-adrenocortical activity in female rhesus monkeys (Macaca mulatta). Horm. Behav. 1993, 27, 318–331. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic stress glucocorticoid receptor resistance inflammation and disease risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef] [Green Version]
- Fagundes, C.P.; Diamond, L.M.; Allen, K.P. Adolescent attachment insecurity and parasympathetic functioning predict future loss adjustment. Pers. Soc. Psychol. Bull. 2012, 38, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; McEwen, B. S Adverse childhood experiences allostasis allostatic load and age-related disease. Physiol. Behav. 2011, 106, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Shugart, M.; Craighead, W.E.; Nemeroff, C.B. Neurobiological and psychiatric consequences of child abuse and neglect. Dev. Psychobiol. 2010, 52, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-related fatigue: Links with inflammation in cancer patients and survivors. Brain Behav. Immun. 2007, 21, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Tabassum, D.P.; Polyak, K. Tumorigenesis: It takes a village. Nat. Rev. Cancer 2015, 15, 473–483. [Google Scholar] [CrossRef]
- Danese, A.; Moffitt, T.E.; Pariante, C.M.; Ambler, A.; Poulton, R.; Caspi, A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch. Gen. Psychiatry 2008, 65, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S.; Garlapati, C.; Aneja, R. Epigenetic Determinants of Racial Disparity in Breast Cancer: Looking beyond Genetic Alterations. Cancers 2022, 14, 1903. [Google Scholar] [CrossRef]
- Krause, B.J.; Artigas, R.; Sciolla, A.F.; and Hamilton, J. Epigenetic mechanisms activated by childhood adversity. Epigenomics 2020, 12, 1239–1255. [Google Scholar] [CrossRef]
- Marzi, S.J.; Sugden, K.; Arseneault, L.; Belsky, D.W.; Burrage, J.; Corcoran, D.L.; Danese, A.; Fisher, H.L.; Hannon, E.; Moffitt, T.E.; et al. Analysis of DNA methylation in young people: Limited evidence for an association between victimization stress and epigenetic variation in blood. Am. J. Psychiatry 2018, 175, 517–529. [Google Scholar] [CrossRef]
- Martins, J.; Czamara, D.; Sauer, S.; Rex-Haffner, M.; Dittrich, K.; Dörr, P.; de Punder, K.; Overfeld, J.; Knop, A.; Dammering, F.; et al. Childhood adversity correlates with stable changes in DNA methylation trajectories in children and converges with epigenetic signatures of prenatal stress. Neurobiol. Stress 2021, 15, 100336. [Google Scholar] [CrossRef]
- Burns, S.B.; Almeida, D.; Turecki, G. The epigenetics of early life adversity: Current limitations and possible solutions. Prog. Mol. Biol. Transl. Sci. 2018, 157, 343–425. [Google Scholar] [CrossRef]
- O’Donnell, K.J.; Chen, L.; Macisaac, J.L.; Mcewen, L.M.; Nguyen, T.; Beckmann, K.; Zhu, Y.; Chen, L.M.; Brooks-Gunn, J.; Goldman, D.; et al. DNA methylome variation in a perinatal nurse-visitation program that reduces child maltreatment: A 27-year follow-up. Transl. Psychiatry 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perroud, N.; Salzmann, A.; Prada, P.; Nicastro, R.; Hoeppli, M.E.; Furrer, S.; Ardu, S.; Krejci, I.; Karege, F.; Malafosse, A. Response to psychotherapy in borderline personality disorder and methylation status of the BDNF gene. Transl. Psychiatry 2013, 3, e207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldwin, J.R.; Reuben, A.; Newbury, J.B.; Danese, A. Agreement between prospective and retrospective measures of childhood maltreatment: A systematic review and meta-analysis. JAMA Psychiat. 2019, 76, 584–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yehuda, R.; Daskalakis, N.P.; Lehrner, A.; Desarnaud, F.; Bader, H.N.; Makotkine, I.; Flory, J.D.; Meaney, M.J. Influences of maternal and paternal PTSD on epigenetic regulation of the glucocorticoid receptor gene in Holocaust survivor offspring. Am. J. Psychiatry 2014, 171, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Sumner, J.A.; Gambazza, S.; Gao, X.; Baccarelli, A.A.; Uddin, M.; McLaughlin, K.A. Epigenetics of early-life adversity in youth: Cross-sectional and longitudinal associations. Clin. Epigenet. 2022, 14, 48. [Google Scholar] [CrossRef] [PubMed]


| Reference | Type of Survey | Sample Size | ACEs | Psychological Tools | Biomedical Measures | Associations | Stage | |
|---|---|---|---|---|---|---|---|---|
| Inflammation biomarkers and psychological measures | Crosswell et al., 2014 [32] | Retrospective, cross-sectional, 1-year post-treatment | 152 (21–65 years old) chosen from a wider cancer study; mean age = 51.7 (7.8) | Risky Family Questionnaire (RFQ) Score 27.75 | (1) BDI 1 (2) PSS 2 | IL-6 3, CRP 4, IL-1ra 5, sTNF-RII 6 | Inflammatory markers and RFQ 7 subscales. Risky factor total score with IL-6 3 marginally associated with CRP 4; abuse and IL-6 3; chaotic environment with IL-6 3 and TNF 8. | 0-IIIA |
| Han et al., 2016 [36] | Retrospective-cohort, prospective longitudinal, 1-week pre-radiation; week 6 of radiation and 6 weeks after | 20; mean age = 53.16 (11.6) | Childhood Trauma Questionnaire (CTQ) | (1) MFI 9 (2) IDS-SR 10 (3) PSS 2 | Gene expression CRP 4, (IL)-6 3, and IL-1ra 5 | Childhood trauma and susceptibility of increased stress, fatigue, and inflammation during breast radiotherapy (RT). | 0-IIIA | |
| Witek-Janusek et al., 2013 [37] | Cohort, retrospective, longitudinal prospective, 5 measures in 9 months | 40 women (34 breast conserving surgery + radiation, 6 just surgery); mean age = 55.6 (9.4) | Childhood Trauma Questionnaire (CTQ) | (1) PSS 2 (2) CES-D 11 (3) MFI 9 (4) QLI 12 | PBMC 13, NKCA 14, IL-6 3 | Childhood adversity factors vs. psychological measures and biomarkers over time. | Stages I, II and IIA (78% stage 0 or I) | |
| Stress biomarker and psychological measures | Kamen et al., 2017 [34] | Cross sectional, retrospective; baseline, 3 weeks (mid-treatment), 6 weeks (end of treatment), 10 weeks follow-up | 56 adult women diagnosed but not currently in treatment; mean of 53.6 years (9.8) | Traumatic Events Survey (TES) | (1) CES-D 11 (2) Charlson index (3) PSQI 15 | Salivary cortisol | (1) ACEs vs. cognitive functioning, cancer treatment, time since treatment, depression, anxiety, and sleep.(2) Cognitive functioning vs. cortisol. | 19.6% stage I 41.1% stage II 16.1% stage III 3.6% stage IV |
| Immunological and psychological measures | Fagundes et al., 2013 [33] | Cross-sectional, retrospective, from previous RCT | 108 with EBV 16 (104) and/or CMV 17 (56) seropositive; mean age = 51.59 (9.39) | Six different conditions (e.g., death of mother or lack of close relationship with adult) | (1) CES-D 11 (2) Charlson index; (3) PSQI 15 | EBV 16, CMV 17 | (1) Relationship between ACEs and depressive symptoms. (2) Relationship between childhood adversities and EBV 16 and CMV 17 antibody titers. | 0-IIIA |
| Bower et al., 2020 [38] | Retrospective, longitudinal | 86 with BS chosen from RISE study; mean age = 55.95 (11.8) | Childhood Trauma Questionnaire (CTQ) categorized in 3 group based on gravity (0–0.5–1) | (1) CES-D 11 | CD14+ monocytes from PBMC, RNA extraction, gene expression analyses | (1) Relationship between ACEs and BC. (2) Relationship between ACEs, NF-κB-binding motifs, and depression. (3) Analyses of Type I interferon. | 0-IIIA | |
| McFarland et al., 2016 [35] | Cohort, retrospective | 125; mean age = 55.36 (13.20) | Risky Family Questionnaire: (1) Abuse; (2) Neglect; (3) Chaotic Home Environment | (1) DT a PL 18 (2) HADS 19-Anxiety; (3) HADS 19-Depression | None | ECA 20 vs. distress, anxiety, and depression. | 0-IV within 5 years of diagnosis | |
| Goldsmith et al., 2010 [21] | Cross-sectional, retrospective | 303 recruited from private and public hospitals; mean age = 50.68 (9.86) | Childhood Trauma Questionnaire (CTQ) | IES 21 | None | Childhood abuse, particularly emotional abuse vs. levels of cancer-related intrusive and avoidant symptoms. | Not specified | |
| Wahbeh et al., 2021 [31] | Cross-sectional, retrospective Online surveys and cross-media measures | Clinical sample of 1041 women with BC (mean age = 57) Control group of 1000 women (mean age = 55.5) | (CTQ) short form + time (0–7; 8–18; 19–90 years old). Item #7 from Adverse Childhood Experience (ACE) questionnaire. Life Events Checklist (LEC). Ad hoc questionnaire. | Post Traumatic Growth Inventory Short-Form (PTGI-SF) | Basal metabolic index, reproductive health, hormone therapy, DES 22 history | Predictors of BC status: childhood trauma frequency (CTQ); trauma type; repetition across life; job experience. | 16.6% (0) 27.8% (I) 27.1% (II) 15.9% (III) 7.2% (IV) 11.0% BRCA 23 + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino, O.; Cadena, R.T.; Poli, D. A Comprehensive Review on Multifaceted Mechanisms Involved in the Development of Breast Cancer Following Adverse Childhood Experiences (ACEs). Int. J. Environ. Res. Public Health 2022, 19, 12615. https://doi.org/10.3390/ijerph191912615
Pino O, Cadena RT, Poli D. A Comprehensive Review on Multifaceted Mechanisms Involved in the Development of Breast Cancer Following Adverse Childhood Experiences (ACEs). International Journal of Environmental Research and Public Health. 2022; 19(19):12615. https://doi.org/10.3390/ijerph191912615
Chicago/Turabian StylePino, Olimpia, Rosalinda Trevino Cadena, and Diana Poli. 2022. "A Comprehensive Review on Multifaceted Mechanisms Involved in the Development of Breast Cancer Following Adverse Childhood Experiences (ACEs)" International Journal of Environmental Research and Public Health 19, no. 19: 12615. https://doi.org/10.3390/ijerph191912615