Chromosome Asynapsis Is the Main Cause of Male Sterility in the Interspecies Hybrids of East Asian Voles (Alexandromys, Rodentia, Arvicolinae)
Abstract
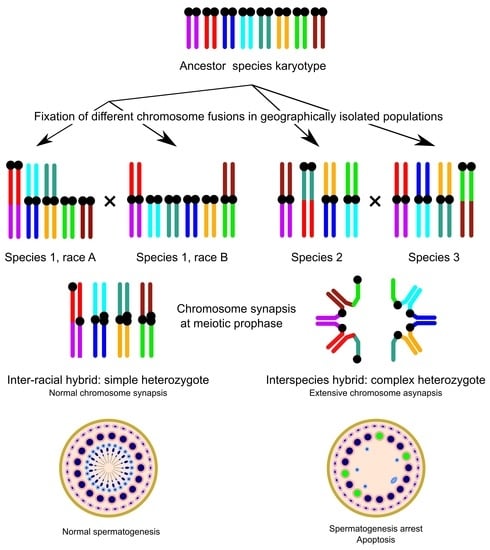
:1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Histological Analysis
2.3. Detection of Apoptotic Cells in Seminiferous Tubules
2.4. SC Spreading for Light and Electron Microscopy
2.5. Data Analysis
3. Results
3.1. Histological Analysis
3.1.1. Parental Species, Interpopulation, and Interracial Hybrids
3.1.2. Interspecies Hybrids
3.2. Cytological Analysis
3.2.1. Parental Species
Alexandromys maximowiczii (MAX)
Alexandromys mujanensis (MUJ)
Alexandromys evoronensis “Argi” (EVA)
Alexandromys evoronensis “Evoron” (EVE)
3.2.2. Interracial Hybrids
3.2.3. Interspecies Hybrids
4. Discussion
4.1. Interspecies Hybrids Are Sterile, Whereas Intraspecies Hybrids Show Normal Reproductive Potential
4.2. Asynapsis Is the Main Cause of Meiotic Arrest and Sterility in Interspecies Hybrids
4.3. Asynapsis in Interspecies Hybrids Is Due to Complex Heterozygosity for a Series of Chromosome Rearrangements
4.4. Simple Heterozygosity for Several Chromosome Rearrangements Does Not Disrupt Chromosome Synapsis and Recombination in the Intraspecies Hybrids of A. evoronensis
4.5. Chromosome Polymorphism of the “Maximowiczii” Group of Species Is Apparently Neutral
4.6. Karyotypic Divergence Promotes Speciation in the “Maximowiczii” Group of Species
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferguson-Smith, M.A.; Trifonov, V. Mammalian karyotype evolution. Nat. Rev. Genet. 2007, 8, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Faria, R.; Navarro, A. Chromosomal speciation revisited: Rearranging theory with pieces of evidence. Trends Ecol. Evol. 2010, 25, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Ayala, F.J.; Coluzzi, M. Chromosome speciation: Humans, Drosophila, and mosquitoes. Proc. Natl. Acad. Sci. USA 2005, 102, 6535–6542. [Google Scholar] [CrossRef]
- Rieseberg, L.H. Chromosomal rearrangements and speciation. Trends Ecol. Evol. 2001, 16, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Butlin, R.K. Recombination and speciation. Mol. Ecol. 2005, 14, 2621–2635. [Google Scholar] [CrossRef]
- Belonogova, N.M.; Polyakov, A.V.; Karamysheva, T.V.; Torgasheva, A.A.; Searle, J.B.; Borodin, P.M. Chromosome synapsis and recombination in male hybrids between two chromosome races of the common shrew (Sorex araneus L., Soricidae, Eulipotyphla). Genes 2017, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Searle, J.B. Meiotic studies of Robertsonian heterozygotes from natural populations of the common shrew, Sorex araneus L. Cytogenet. Genome Res. 1986, 41, 154–162. [Google Scholar] [CrossRef]
- Borodin, P.M.; Fedyk, S.; Chętnicki, W.; Torgasheva, A.A.; Pavlova, S.V.; Searle, J.B. Meiosis and fertility associated with chromosomal heterozygosity. In Shrews, Chromosomes and Speciation; Zima, J., Searle, J.B., Polly, P.D., Eds.; Cambridge Studies in Morphology and Molecules: New Paradigms in Evolutionary Bio; Cambridge University Press: Cambridge, UK, 2019; pp. 217–270. ISBN 9781107011373. [Google Scholar]
- King, M. Species Evolution: The Role of Chromosome Change; Cambridge University Press: Cambridge, UK, 1993; ISBN 0521484545. [Google Scholar]
- Bakloushinskaya, I. Chromosome changes in soma and germ line: Heritability and evolutionary outcome. Genes 2022, 13, 602. [Google Scholar] [CrossRef]
- Forejt, J. Meiotic studies of translocations causing male sterility in the mouse. Cytogenet. Genome Res. 1979, 23, 163–170. [Google Scholar] [CrossRef]
- Garagna, S.; Page, J.; Fernandez-Donoso, R.; Zuccotti, M.; Searle, J.B. The Robertsonian phenomenon in the house mouse: Mutation, meiosis and speciation. Chromosoma 2014, 123, 529–544. [Google Scholar] [CrossRef]
- Forejt, J.; Jansa, P. Meiotic recognition of evolutionarily diverged homologs: Chromosomal hybrid sterility revisited. Mol. Biol. Evol. 2023, 40, msad083. [Google Scholar] [CrossRef] [PubMed]
- Everett, C.A.; Searle, J.B.; Wallace, B.M. A study of meiotic pairing, nondisjunction and germ cell death in laboratory mice carrying Robertsonian translocations. Genet. Res. 1996, 67, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Matveevsky, S.N.; Kolomiets, O.L. Synaptonemal complex configurations in Robertsonian heterozygotes. Tsitologiia 2016, 58, 309–314. [Google Scholar] [PubMed]
- Microtus maximowiczii. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/species/13442/115113061 (accessed on 24 April 2023).
- Kryštufek, B.; Shenbrot, G. Voles and Lemmings (Arvicolinae) of the Palaearctic Region; University of Maribor Press: Maribor, Slovenia, 2022; ISBN 9789612866112. [Google Scholar]
- Kartavtseva, I.V.; Sheremetyeva, I.N.; Korobitsina, K.V.; Nemkova, G.A.; Konovalova, E.V.; Korablev, V.V.; Voyta, L.L. Chromosomal forms of Microtus maximowiczii (Schrenck, 1859) (Rodentia, Cricetidae): Variability in 2n and NF in different geographic regions. Russ. J. Theriol. 2008, 7, 89–97. [Google Scholar] [CrossRef]
- Lemskaya, N.A.; Romanenko, S.A.; Golenishchev, F.N.; Rubtsova, N.V.; Sablina, O.V.; Serdukova, N.A.; O’Brien, P.C.M.; Fu, B.; Yiğit, N.; Ferguson-Smith, M.A.; et al. Chromosomal evolution of Arvicolinae (Cricetidae, Rodentia). III. Karyotype relationships of ten Microtus species. Chromosome Res. 2010, 18, 459–471. [Google Scholar] [CrossRef]
- Golenishchev, F.N.; Voyta, L.L.; Moroldoev, I.V.; Abramson, N.I.; Petrova, T.V.; Kartavtseva, I.V. New transbaikalian finds of the Muja valley vole (Rodentia: Cricetidae: Alexandromys mujanensis). Proc. Zool. Inst. RAS 2018, 322, 357–384. [Google Scholar] [CrossRef]
- Meyer, M.N.; Golenishchev, F.N.; Radjably, S.I.; Sablina, O.V. Voles (Subgenus Microtus Schrank) of Russia and Adjacent Territories; Zoological Institute of RAS: Sankt-Petersburg, Russia, 1996. [Google Scholar]
- Orlov, V.N.; Kovalskaya, Y.M. Microtus mujanensis sp. n. from the Vitim River Basin. Zool. Zhurnal 1978, 57, 1224–1232. [Google Scholar]
- Lemskaya, N.A.; Kartavtseva, I.V.; Rubtsova, N.V.; Golenishchev, F.N.; Sheremetyeva, I.N.; Graphodatsky, A.S. Chromosome polymorphism in Microtus (Alexandromys) mujanensis (Arvicolinae, Rodentia). Cytogenet. Genome Res. 2015, 146, 238–242. [Google Scholar] [CrossRef]
- Kartavtseva, I.V.; Vasilieva, T.V.; Sheremetyeva, I.N.; Lemskaya, N.A.; Moroldoev, I.V.; Golenishchev, F.N. Genetic variability of three isolated populations of the Muya valley vole Alexandromys mujanensis Orlov et Kovalskaja, 1978 (Rodentia, Arvicolinae). Russ. J. Genet. 2019, 55, 978–992. [Google Scholar] [CrossRef]
- Kovalskaya, Y.M.; Sokolov, V.E. A new vole species (Rodentia, Cricetidae, Microtus) from the Lower Amur region. Zoololog. J. 1980, 59, 1409–1416. [Google Scholar]
- Sheremetyeva, I.N.; Kartavtseva, I.V.; Vasiljeva, T.V. Does Alexandromys evoronensis inhabit the northeastern part of Verkhnezeiskaya Plain? Biol. Bull. 2017, 44, 1151–1157. [Google Scholar] [CrossRef]
- Kartavtseva, I.V.; Sheremetyeva, I.N.; Pavlenko, M.V. Multiple chromosomal polymorphism of “Evoron” chromosomal race of the Evoron vole (Rodentia, Arvicolinae). Russ. J. Genet. 2021, 57, 70–82. [Google Scholar] [CrossRef]
- Kartavtseva, I.V.; Sheremetyeva, I.N.; Pavlenko, M.V. Intraspecies multiple chromosomal variations including rare tandem fusion in the Russian Far Eastern endemic Evoron vole Alexandromys evoronensis (Rodentia, Arvicolinae). Comp. Cytogenet. 2021, 15, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Bannikova, A.A.; Lebedev, V.S.; Lissovsky, A.A.; Matrosova, V.; Abramson, N.I.; Obolenskaya, E.V.; Tesakov, A.S. Molecular phylogeny and evolution of the Asian lineage of vole genus Microtus (Rodentia: Arvicolinae) inferred from mitochondrial cytochrome b sequence. Biol. J. Linn. Soc. 2010, 99, 595–613. [Google Scholar] [CrossRef]
- Bannikova, A.A.; Lebedev, V.S.; Poplavskaya, N.S.; Simanovsky, S.A.; Undrakhbayar, E.; Adiya, Y.; Surov, A.S. Phylogeny and phylogeography of arvicoline and lagurine voles of Mongolia. Folia Zool. 2019, 68, 100–113. [Google Scholar] [CrossRef]
- Meyer, M.N. Hybridization. Breeding intensity. In Common Vole: Sibling Species; Sokolov, V.E., Bashenina, N.V., Eds.; Nauka: Moscow, Russia, 1994; pp. 26–32. [Google Scholar]
- Anderson, L.K.; Reeves, A.; Webb, L.M.; Ashley, T. Distribution of crossing over on mouse synaptonemal complexes using immunofluorescent localization of MLH1 protein. Genetics 1999, 151, 1569–1579. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef]
- Turner, J.M.A. Meiotic silencing in mammals. Annu. Rev. Genet. 2015, 49, 395–412. [Google Scholar] [CrossRef]
- Russell, L.D.; Ettlin, R.A.; Hikim, A.P.S.; Clegg, E.D. Histological and histopathological evaluation of the testis. Int. J. Androl. 1993, 16, 83. [Google Scholar] [CrossRef]
- Ahmed, E.A.; de Rooij, D.G. Staging of mouse seminiferous tubule cross-sections. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; Volume 558, pp. 263–277. [Google Scholar]
- Galleni, L.; Tellini, A.; Cicalò, A.; Fabiani, C.; Fabiani, O. Histological examination of the male gonad of hybrid specimens: Microtus savii x M.brachycercus (Rodentia-Arvicolinae). Bonner Zool. Beiträge 1998, 48, 1–7. [Google Scholar]
- Peters, A.H.F.M.; Plug, A.W.; van Vugt, M.J.; de Boer, P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosom. Res. 1997, 5, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Howell, W.M.; Black, D.A. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: A 1-step method. Experientia 1980, 36, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: www.R-project.org/ (accessed on 24 April 2023).
- Poorman, P.A.; Moses, M.J.; Davisson, M.T.; Roderick, T.H. Synaptonemal complex analysis of mouse chromosomal rearrangements. III. Cytogenetic observations on two paracentric inversions. Chromosoma 1981, 83, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Moses, M.J.; Poorman, P.A.; Roderick, T.H.; Davisson, M.T. Synaptonemal complex analysis of mouse chromosomal rearrangements. IV. Synapsis and synaptic adjustment in two paracentric inversions. Chromosoma 1982, 84, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Torgasheva, A.A.; Borodin, P.M. Synapsis and recombination in inversion heterozygotes. Biochem. Soc. Trans. 2010, 38, 1676. [Google Scholar] [CrossRef] [PubMed]
- Borodin, P.M.; Rodionova, M.I.; Sablina, O.V.; Gorlov, I.P. Unusual heteromorphic bivalents in the common vole (Microtus arvalis) from Belorussia. Cytogenet. Genome Res. 1992, 60, 123–127. [Google Scholar] [CrossRef]
- Turner, J.M.; Mahadevaiah, S.K.; Fernandez-Capetillo, O.; Nussenzweig, A.; Xu, X.; Deng, C.X. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat. Genet. 2005, 37, 41–47. [Google Scholar] [CrossRef]
- Burgoyne, P.S.; Mahadevaiah, S.K.; Turner, J.M. The consequences of asynapsis for mammalian meiosis. Nat. Rev. Genet. 2009, 10, 207–216. [Google Scholar] [CrossRef]
- Odorisio, T.; Rodriguez, T.A.; Evans, E.P.; Clarke, A.R.; Burgoyne, P.S. The meiotic checkpoint monitoring synapsis eliminates spermatocytes via p53-independent apoptosis. Nat. Genet. 1998, 18, 257–261. [Google Scholar] [CrossRef]
- Cloutier, J.M.; Turner, J.M.A. Meiotic sex chromosome inactivation. Curr. Biol. 2010, 20, R962–R963. [Google Scholar] [CrossRef]
- Garagna, S.; Zuccotti, M.; Thornhill, A.; Fernandez-Donoso, R.; Berrios, S.; Capanna, E.; Redi, C.A. Alteration of nuclear architecture in male germ cells of chromosomally derived subfertile mice. J. Cell Sci. 2001, 114, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Medarde, N.; Merico, V.; López-Fuster, M.J.; Zuccotti, M.; Garagna, S.; Ventura, J. Impact of the number of Robertsonian chromosomes on germ cell death in wild male house mice. Chromosom. Res. 2015, 23, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Merico, V.; Pigozzi, M.I.; Esposito, A.; Merani, M.S.; Garagna, S. Meiotic recombination and spermatogenic impairment in Mus musculus domesticus carrying multiple simple Robertsonian translocations. Cytogenet. Genome Res. 2003, 103, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Searle, J.B. Chromosomal hybrid zones in eutherian mammals. In Hybrid Zones and the Evolutionary Process; Harrison, R.G., Ed.; Oxford University Press: New York, NY, USA, 1993; pp. 309–353. [Google Scholar]
- Förster, D.W.; Jones, E.P.; Jóhannesdóttir, F.; Gabriel, S.I.; Giménez, M.D.; Panithanarak, T.; Hauffe, H.C.; Searle, J.B. Genetic differentiation within and away from the chromosomal rearrangements characterising hybridising chromosomal races of the western house mouse (Mus musculus domesticus). Chromosom. Res. 2016, 24, 271–280. [Google Scholar] [CrossRef] [PubMed]
- de Boer, P.; de Jong, J.H. Chromosome Pairing and Fertility in Mice. In Fertility and Chromosome Pairing: Recent Studies in Plants and Animals; Gillies, C.B., Ed.; CRC Press: Boca Raton, FL, USA, 1989; pp. 37–76. [Google Scholar]
- de Boer, P.; Searle, A.G.; van der Hoeven, F.A.; de Rooij, D.G.; Beechey, C.V. Male pachytene pairing in single and double translocation heterozygotes and spermatogenic impairment in the mouse. Chromosoma 1986, 93, 326–336. [Google Scholar] [CrossRef]
- Redi, C.A.; Garagna, S.; Hilscher, B.; Winking, H. The effects of some Robertsonian chromosome combinations on the seminiferous epithelium of the mouse. J. Embryol. Exp. Morphol. 1985, 85, 1–19. [Google Scholar] [CrossRef]
- Hauffe, H.C.; Searle, J.B. Chromosomal heterozygosity and fertility in house mice (Mus musculus domesticus) from Northern Italy. Genetics 1998, 150, 1143–1154. [Google Scholar] [CrossRef]
- Ribagorda, M.; Berríos, S.; Solano, E.; Ayarza, E.; Martín-Ruiz, M.; Gil-Fernández, A.; Parra, M.T.; Viera, A.; Rufas, J.S.; Capanna, E.; et al. Meiotic behavior of a complex hexavalent in heterozygous mice for Robertsonian translocations: Insights for synapsis dynamics. Chromosoma 2019, 128, 149–163. [Google Scholar] [CrossRef]
- Lanzone, C.; Gimenez, M.D.; Santos, J.L.; Bidau, C.J. Meiotic effects of Robertsonian translocations in tuco-tucos of the Ctenomys perrensi superspecies (Rodentia: Ctenomyidae). Caryolgia 2007, 60, 233–244. [Google Scholar] [CrossRef]
- Basheva, E.A.; Torgasheva, A.A.; Gomez Fernandez, M.J.; Boston, E.; Mirol, P.; Borodin, P.M. Chromosome synapsis and recombination in simple and complex chromosomal heterozygotes of tuco-tuco (Ctenomys talarum: Rodentia: Ctenomyidae). Chromosom. Res. 2014, 22, 351–363. [Google Scholar] [CrossRef]
- Wallace, B.M.N.; Searle, J.B.; Everett, C.A. The effect of multiple simple Robertsonian heterozygosity on chromosome pairing and fertility of wild-stock house mice (Mus musculus domesticus). Cytogenet. Genome Res. 2002, 96, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Borodin, P.M.; Basheva, E.A.; Torgasheva, A.A.; Golenishchev, F.N.; Kartavtseva, I.V. Synapsis and chromosome recombination in the Muya vole (Microtus mujanensis). Vestn. NGU. Ser. Biol. Clin. Med. 2010, 8, 124–130. [Google Scholar]
- Bikchurina, T.I.; Golenishchev, F.N.; Kizilova, E.A.; Mahmoudi, A.; Borodin, P.M. Reproductive isolation between taxonomically controversial forms of the gray voles (Microtus, Rodentia; Arvicolinae): Cytological mechanisms and taxonomical implications. Front. Genet. 2021, 12, 653837. [Google Scholar] [CrossRef] [PubMed]
- Torgasheva, A.A.; Borodin, P.M. Cytological basis of sterility in male and female hybrids between sibling species of grey voles Microtus arvalis and M. levis. Sci. Rep. 2016, 6, 36564. [Google Scholar] [CrossRef] [PubMed]
- Schwander, T.; Libbrecht, R.; Keller, L. Supergenes and complex phenotypes. Curr. Biol. 2014, 24, R288–R294. [Google Scholar] [CrossRef] [PubMed]
- Bakloushinskaya, I.Y.; Matveevsky, S.N.; Romanenko, S.A.; Serdukova, N.A.; Kolomiets, O.L.; Spangenberg, V.E.; Lyapunova, E.A.; Graphodatsky, A.S. A comparative analysis of the mole vole sibling species Ellobius tancrei and E. talpinus (Cricetidae, Rodentia) through chromosome painting and examination of synaptonemal complex structures in hybrids. Cytogenet. Genome Res. 2012, 136, 199–207. [Google Scholar] [CrossRef]
- Nevo, E.; Filippucci, M.G.; Redi, C.; Korol, A.; Beiles, A. Chromosomal speciation and adaptive radiation of mole rats in Asia Minor correlated with increased ecological stress. Proc. Natl. Acad. Sci. USA 1994, 91, 8160–8164. [Google Scholar] [CrossRef]
- Torgasheva, A.A.; Basheva, E.A.; Gomez Fernandez, M.J.; Mirol, P.; Borodin, P.M. Chromosomes and speciation in tuco-tuco (Ctenomys, Hystricognathi, Rodentia). Vavilov J. Genet. Breed. 2016, 20, 408–415. [Google Scholar] [CrossRef]
- Matveevsky, S.; Kolomiets, O.; Bogdanov, A.; Alpeeva, E.; Bakloushinskaya, I. Meiotic chromosome contacts as a plausible prelude for robertsonian translocations. Genes 2020, 11, 386. [Google Scholar] [CrossRef]
- Mazurok, N.A.; Rubtsova, N.V.; Isaenko, A.A.; Pavlova, M.E.; Ya, S.; Nesterova, T.B.; Zakian, S.M.; Slobodyanyuk, S.Y.; Nesterova, T.B.; Zakian, S.M. Comparative chromosome and mitochondrial DNA analyses and phylogenetic relationships within common voles (Microtus, Arvicolidae). Chromosom. Res. 2001, 9, 107–120. [Google Scholar] [CrossRef]





| Dam | Sire | N | Most Advanced Stage of Spermatogenesis | Spermatogenesis | Meiotic Prophase | Mean Number of MLH1 Foci per Cell |
|---|---|---|---|---|---|---|
| Parental crosses | ||||||
| MAX | MAX | 4 | Spermatozoa | Normal | Normal | 23.9 ± 2.2 |
| MUJ | MUJ | 3 | Spermatozoa | Normal | Normal | 20.2 ± 1.6 |
| EVA | EVA | 4 | Spermatozoa | Normal | Normal | 20.7 ± 2.0 |
| EVE | EVE | 2 | Spermatozoa | Normal | Normal | 22.0 ± 1.1 |
| Interpopulation and interracial crosses | ||||||
| EVA2 | EVA1 | 2 | Spermatozoa | Normal | Normal | 20.5 ± 1.5 |
| EVA | EVE | 3 | Spermatozoa in two males, early spermatocytes I in one male | Normal in two males, delayed spermatogenesis in one male | Normal | 21.1 ± 1.3 |
| Interspecies crosses | ||||||
| MUJ | MAX | 1 | Spermatozoa | Spermatid balls, abnormal spermatozoa | Univalents, heteromorphic, and partially asynaptic bivalents, complex multivalents, γH2A.X at asynapsed regions, MLH1 foci at each synapsed element | 21.3 ± 3.3 |
| EVA | MUJ | 2 | Spermatozoa | Spermatid balls and abnormal spermatozoa in one male, sporadic normal spermatids in another male | Univalents, heteromorphic, and partially asynaptic bivalents, complex multivalents, γH2A.X at the asynapsed regions, few MLH1 foci. | - |
| MUJ | EVA | 1 | Spermatozoa | Arrest at a pachytene-like stage | Univalents, heteromorphic, and partially asynaptic bivalents, complex multivalents, γH2A.X at the asynapsed regions, no MLH1 foci. | - |
| MAX | EVE | 3 | Early spermatocytes I | Arrest at a pachytene-like stage | Univalents, heteromorphic, and partially asynaptic bivalents, complex multivalents, γH2A.X at the asynapsed regions, no MLH1 foci. | - |
| EVA | MAX | 4 | Early spermatocytes I | Arrest at zygotene stage mostly | Univalents, heteromorphic, and partially asynaptic bivalents, multivalents with extended asynapsis, γH2A.X at the asynapsed regions, MLH1 foci detected in 4 cells. | 23.3 ± 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikchurina, T.; Pavlenko, M.; Kizilova, E.; Rubtsova, D.; Sheremetyeva, I.; Kartavtseva, I.; Torgasheva, A.; Borodin, P. Chromosome Asynapsis Is the Main Cause of Male Sterility in the Interspecies Hybrids of East Asian Voles (Alexandromys, Rodentia, Arvicolinae). Genes 2023, 14, 1022. https://doi.org/10.3390/genes14051022
Bikchurina T, Pavlenko M, Kizilova E, Rubtsova D, Sheremetyeva I, Kartavtseva I, Torgasheva A, Borodin P. Chromosome Asynapsis Is the Main Cause of Male Sterility in the Interspecies Hybrids of East Asian Voles (Alexandromys, Rodentia, Arvicolinae). Genes. 2023; 14(5):1022. https://doi.org/10.3390/genes14051022
Chicago/Turabian StyleBikchurina, Tatiana, Marina Pavlenko, Elena Kizilova, Daria Rubtsova, Irina Sheremetyeva, Irina Kartavtseva, Anna Torgasheva, and Pavel Borodin. 2023. "Chromosome Asynapsis Is the Main Cause of Male Sterility in the Interspecies Hybrids of East Asian Voles (Alexandromys, Rodentia, Arvicolinae)" Genes 14, no. 5: 1022. https://doi.org/10.3390/genes14051022