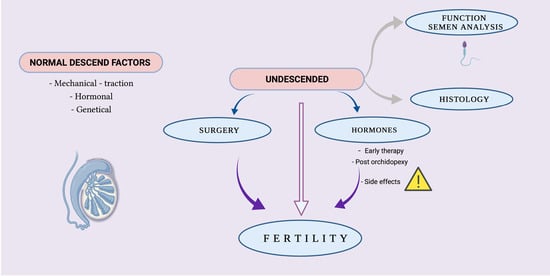
Fertility of Cryptorchid Testis—An Unsolved Mistery
Abstract
:1. Introduction
2. Factors Controlling Normal Descending of the Testis
3. Cryptorchidism Impact on Testicular Function and on Semen Analysis
4. Cryptorchidism Impact on Testicular Histology
5. Cryptorchidism Early Surgical Therapy and Its Implication on Fertility
6. Cryptorchidism Hormonal Therapy and Its Implication on Fertility
6.1. Early Hormonal Therapy
6.2. Post Orchidopexy GnRh Therapy
6.3. Adverse Effect of Hormone Therapy on Future Fertility
7. Cryptorchidism and Its Implication on Infertility Management and Paternity
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serrano, T.; Chevrier, C.; Multigner, L.; Cordier, S.; Jegou, B. International geographic correlation study of the prevalence of disorders of male reproductive health. Hum. Reprod. 2013, 28, 1974–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swee, D.S.; Quinton, R. Congenital Hypogonadotrophic Hypogonadism: Minipuberty and the Case for Neonatal Diagnosis. Front. Endocrinol. 2019, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.A.; Coughlin, M.T. Fertility after Bilateral Cryptorchidism. Horm. Res. Paediatr. 2001, 55, 28–32. [Google Scholar] [CrossRef]
- Hadziselimovic, F.; Herzog, B. The importance of both an early orchidopexy and germ cell maturation for fertility. Lancet 2001, 358, 1156–1157. [Google Scholar] [CrossRef]
- Hutson, J.; Baker, M.; Terada, M.; Zhou, B.; Paxton, G. Hormonal control of testicular descent and the cause of cryptorchidism. Reprod. Fertil. Dev. 1994, 6, 151–156. [Google Scholar] [CrossRef]
- Zimmermann, S.; Steding, G.; Emmen, J.M.; Brinkmann, A.O.; Nayernia, K.; Holstein, A.F.; Engel, W.; Adham, I.M. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol. Endocrinol. 1999, 13, 681–691. [Google Scholar] [CrossRef]
- Heyns, C.; Human, H.; Werely, C.; De Klerk, D. The Glycosaminoglycans of the Gubernaculum During Testicular Descent in the Fetus. J. Urol. 1990, 143, 612–617. [Google Scholar] [CrossRef]
- Hutson, J.M.; Hasthorpe, S.; Heyns, C.F. Anatomical and Functional Aspects of Testicular Descent and Cryptorchidism*. Endocr. Rev. 1997, 18, 259–280. [Google Scholar] [CrossRef]
- Bay, K.; Cohen, A.S.; Jørgensen, F.S.; Jørgensen, C.; Lind, A.M.; Skakkebæk, N.E.; Andersson, A.-M. Insulin-Like Factor 3 Levels in Second-Trimester Amniotic Fluid. J. Clin. Endocrinol. Metab. 2008, 93, 4048–4051. [Google Scholar] [CrossRef] [Green Version]
- Hutson, J.M. Testicular descent. In Descent of the Testis, 2nd ed.; Hutson, J.M., Thorup, J.M., Spencer, W.B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 17–44. [Google Scholar]
- Hadziselimovic, F. Embryology of testicular descent and maldescent. In Cryptorchidism Management and Implications; Hadziselimovic, F., Kogan, S., Cromie, W., Hinman, F., Eds.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 11–53. [Google Scholar]
- Hutson, J.M.; Terada, M.; Zhou, B.; Williams, M.P.L. Normal Testicular Descent and the Aetiology of Cryptorchidism. Adv. Anat. Embryol. Cell Biol. 1996, 132, 1–56. [Google Scholar] [CrossRef]
- Kaplan, L.M.; Koyle, M.A.; Kaplan, G.W.; Farrer, J.H.; Rajfer, J. Association Between Abdominal Wall Defects and Cryptorchidism. J. Urol. 1986, 136, 645–647. [Google Scholar] [CrossRef]
- Hanerhoff, B.L.; Welliver, C. Does early orchidopexy improve fertility? Transl. Androl. Urol. 2014, 3, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Hadziselimovic, F. (Ed.) Cryptorchidism, Management and Implications; Springer: New York, NY, USA, 1983. [Google Scholar]
- Hadziselimovic, F.; Gegenschatz-Schmid, K.; Verkauskas, G.; Demougin, P.; Bilius, V.; Dasevicius, D.; Stadler, M.B. GnRHa Treatment of Cryptorchid Boys Affects Genes Involved in Hormonal Control of the HPG Axis and Fertility. Sex Dev. 2017, 11, 126–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virtanen, H.E.; Bjerknes, R.; Cortes, D.; Jørgensen, N.; Meyts, E.R.-D.; Thorsson, A.V.; Thorup, J.; Main, K.M. Cryptorchidism: Classification, prevalence and long-term consequences. Acta Paediatr. 2007, 96, 611–616. [Google Scholar] [CrossRef]
- Smith, R.; Kaune, H.; Parodi, D.; Madariaga, M.; Morales, I.; Rios, R.; Castro, A. Extent of sperm DNA damage in spermatozoa from men examined for infertility. Relationship with oxidative stress. Revista Medica de Chile 2007, 135, 279–286. (In Spanish) [Google Scholar]
- Hadziselimovic, F. Is Hormonal Treatment of Congenital Undescended Testes Justified? A Debate. Sex. Dev. 2019, 13, 3–10. [Google Scholar] [CrossRef]
- Hadziselimovic, F.; Hadziselimovic, N.; Demougin, P.; Krey, G.; Oakeley, E. Deficient Expression of Genes Involved in the Endogenous Defense System against Transposons in Cryptorchid Boys with Impaired Mini-Puberty. Sex. Dev. 2011, 5, 287–293. [Google Scholar] [CrossRef]
- Hadziselimovic, F.; Hadziselimovic, N.O.; Demougin, P.; Krey, G.; Oakeley, E. Piwi-Pathway Alteration Induces LINE-1 Transposon Derepression and Infertility Development in Cryptorchidism. Sex. Dev. 2015, 9, 98–104. [Google Scholar] [CrossRef]
- Hines, M.; Spencer, D.; Kung, K.T.; Browne, W.V.; Constantinescu, M.; Noorderhaven, R.M. The early postnatal period, mini-puberty, provides a window on the role of testosterone in human neurobehavioural development. Curr. Opin. Neurobiol. 2016, 38, 69–73. [Google Scholar] [CrossRef]
- Cortes, D. Review Cryptorchidism-aspects of pathogenesis, histology and treatment. Scand. J. Urol. Nephrol. Suppl. 1998, 196, 1–54. [Google Scholar]
- Hadziselimovic, F.; Hoecht, B. Testicular histology related to fertility outcome and postpubertal hormone status in cryptorchidism. Klin. Paediatr. 2008, 220, 302–307. [Google Scholar] [CrossRef]
- Gendrel, D.; Roger, M.; Job, J.-C. Plasma gonadotropin and testosterone values in infants with cryptorchidism. J. Pediatr. 1980, 97, 217–220. [Google Scholar] [CrossRef]
- Tasian, G.E.; Hittelman, A.B.; Kim, G.E.; Di Sandro, M.J.; Baskin, L.S. Age at Orchiopexy and Testis Palpability Predict Germ and Leydig Cell Loss: Clinical Predictors of Adverse Histological Features of Cryptorchidism. J. Urol. 2009, 182, 704–709. [Google Scholar] [CrossRef]
- Suskind, A.; Hayner-Buchan, A.; Feustel, P.; Kogan, B.A. Fibrosis correlates with detailed histological analysis of human undescended testes. BJU Int. 2008, 101, 1441–1445. [Google Scholar] [CrossRef]
- Park, K.H.; Lee, J.H.; Han, J.J.; Lee, S.D.; Song, S.Y. Histological evidences suggest recommending orchiopexy within the first year of life for children with unilateral inguinal cryptorchid testis. Int. J. Urol. 2007, 14, 616–621. [Google Scholar] [CrossRef]
- Krishna, O.H.R.; Kotaiah, M.T.; Kota, R.R. Cryptorchidism and its Effects on Histomorphology of Testis in Paediatric Age Group. J. Evol. Med. Dent. Sci. 2019, 8, 2480–2484. [Google Scholar] [CrossRef]
- Huff, D.S.; Fenig, D.M.; Canning, D.A.; Carr, M.G.; Zderic, S.A.; Snyder, H.M. Abnormal germ cell development in cryptorchidism. Horm. Res. 2001, 55, 11–17. [Google Scholar] [CrossRef]
- Schindler, A.M.; Diaz, P.; Cuendet, A.; Sizonenko, P.C. Cryptorchidism: A morphological study of 670 biopsies. Helvetica Paediatr. Acta 1987, 42, 145–158. [Google Scholar]
- Nistal, M.; Paniagua, R.; Riestra, M.L.; Reyes-Múgica, M.; Cajaiba, M.M. Bilateral prepubertal testicular biopsies predict significance of cryptorchidism-associated mixed testicular atrophy, and allow assessment of fertility. Am. J. Surg. Pathol. 2007, 31, 1269–1276. [Google Scholar] [CrossRef]
- Hadžiselimović, F.; Hadziselimovic, N.; Demougin, P.; Oakeley, E. Testicular Gene Expression in Cryptorchid Boys at Risk of Azoospermia. Sex. Dev. 2011, 5, 49–59. [Google Scholar] [CrossRef]
- Tekgül, S.; Stein, R.; Bogaert, G.; Nijman, R.J.; Quaedackers, J.; Silay, M.S.; Radmayr, C.; Doğan, H.S. European Association of Urology/European Society for Paediatric Urology Guidelines. Guidelines Associates: L.A. ’t Hoen, J. Quaedackers, N. Bhatt. Paediatr. Urol. Available online: https://uroweb.org/guideline/paediatric-urology/#3_2 (accessed on 22 November 2021).
- Wenzler, D.L.; Bloom, D.A.; Park, J.M. What is the Rate of Spontaneous Testicular Descent in Infants with Cryptorchidism? J. Urol. 2004, 171, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Radmayr, C.; Dogan, H.S.; Hoebeke, P.; Kočvara, R.; Nijman, R.; Stein, R.; Undre, S.; Tekgul, S. Management of undescended testes: European Association of Urology/European Society for Paediatric Urology Guidelines. J. Pediatr. Urol. 2016, 12, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, G.; Potempa, J. Optimal time for treating cryptorchism (author’s transl). Dtsch. Med. Wochenschr. 1975, 100, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Bica, D.T.; Hadziselimovic, F. Buserelin Treatment of Cryptorchidism: A Randomized, Double-Blind, Placebo-Controlled Study. J. Urol. 1992, 148, 617–621. [Google Scholar] [CrossRef]
- Bica, D.T.; Hadziselimovic, F. The behavior of epididymis, processus vaginalis and testicular descent in cryptorchid boys treated with buserelin. Eur. J. Nucl. Med. Mol. Imaging 1993, 152, S38–S42. [Google Scholar] [CrossRef]
- Hadziselimovic, F. On the descent of the epididymo-testicular unit, cryptorchidism, and prevention of infertility. Basic Clin. Androl. 2017, 27, 1–16. [Google Scholar] [CrossRef]
- Zivkovic, D.; Bica, D.T.; Hadziselimovic, F. Relationship between adult dark spermatogonia and secretory capacity of Leydig cells in cryptorchidism. BJU Int. 2007, 100, 1147–1149. [Google Scholar] [CrossRef]
- Cortes, D.; Thorup, J.; Visfeldt, J. Hormonal treatment may harm the germ cells in 1 to 3-year-old boys with cryptorchidism. J. Urol. 2000, 163, 1290–1292. [Google Scholar] [CrossRef]
- Vinardi, S.; Magro, P.; Manenti, M.; Lala, R.; Costantino, S.; Cortese, M.; Canavese, F. Testicular function in men treated in childhood for undescended testes. J. Pediatr. Surg. 2001, 36, 385–388. [Google Scholar] [CrossRef]
- Zivkovic, D.; Bica, D.; Hadžiselimović, F. Effects of hormonal treatment on the contralateral descended testis in unilateral cryptorchidism. J. Pediatr. Urol. 2006, 2, 468–472. [Google Scholar] [CrossRef]
- Hadziselimovic, F.; Herzog, B. Treatment with a Luteinizing Hormone-Releasing Hormone Analogue After Successful Orchiopexy Markedly Improves the Chance of Fertility Later in Life. J. Urol. 1997, 158, 1195. [Google Scholar] [CrossRef]
- Hadziselimovic, F. Successful treatment of unilateral cryptorchid boys risking infertility with LH-RH analogue. Int. Braz. J. Urol. 2008, 34, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Biers, S.; Malone, P. A critical appraisal of the evidence for improved fertility indices in undescended testes after gonadotrophin-releasing hormone therapy and orchidopexy. J. Pediatr. Urol. 2010, 6, 239–246. [Google Scholar] [CrossRef]
- Assmus, M.; Svechnikov, K.; von Euler, M.; Setchell, B.; Sultana, T.; Zetterström, C.; Holst, M.; Kiess, W.; Söder, O. Single Subcutaneous Administration of Chorionic Gonadotropin to Rats Induces a Rapid and Transient Increase in Testicular Expression of Pro-Inflammatory Cytokines. Pediatr. Res. 2005, 57, 896–901. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekharam, V.V.; Srinivas, M.; Das, S.N.; Jha, P.; Bajpai, M.; Chaki, S.P.; Misro, M.M. Prepubertal human chorionic gonadotropin injection affects postpubertal germ cell maturation and androgen production in rat testis. Urology 2003, 62, 571–574. [Google Scholar] [CrossRef]
- Heiskanen, P.; Billig, H.; Toppari, J.; Kaleva, M.; Arsalo, A.; Rapola, J.; Dunkel, L. Apoptotic Cell Death in the Normal and Cryptorchid Human Testis: The Effect of Human Chorionic Gonadotropin on Testicular Cell Survival. Pediatr. Res. 1996, 40, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Dunkel, L.; Taskinen, S.; Hovatta, O.; Tilly, J.L.; Wikström, S. Germ cell apoptosis after treatment of cryptorchidism with human chorionic gonadotropin is associated with impaired reproductive function in the adult. J. Clin. Investig. 1997, 100, 2341–2346. [Google Scholar] [CrossRef]
- Negri, L.; Albani, E.; DiRocco, M.; Morreale, G.; Novara, P.; Levi-Setti, P.E. Testicular sperm extraction in azoospermic men submitted to bilateral orchidopexy. Hum. Reprod. 2003, 18, 2534–2539. [Google Scholar] [CrossRef] [Green Version]
- Nistal, M.; Riestra, M.L.; Paniagua, R. Focal orchitis in undescended testes: Discussion of pathogenetic mechanisms of tubular atrophy. Arch. Pathol. Lab. Med. 2002, 126, 64–69. [Google Scholar] [CrossRef]
- D’Agostino, S.; Campobasso, P.; Spata, F.; Belloli, G. Cryptorchidism: Anomalies of the secretory ducts and azoospermia. La Pediatr. Medica e Chir. 1996, 18, 41–44. [Google Scholar]
- Gill, B.; Kogan, S.; Starr, S.; Reda, E.; Levitt, S. Significance of Epididymal and Ductal Anomalies Associated with Testicular Maldescent. J. Urol. 1989, 142, 556–558. [Google Scholar] [CrossRef]
- Adamsen, S.; Börjesson, B. Factors affecting the outcome of orchiopexy for undescended testis. Acta Chir. Scand. 1988, 154, 529–533. [Google Scholar] [PubMed]
- Chung, E.; Brock, G.B. Cryptorchidism and its impact on male fertility: A state of art review of current literature. Can. Urol. Assoc. J. 2011, 5, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Comhaire, A.M.; Abdel-Rahim, D.E.; Abdel-Hafez, K.M. Conception rates and assisted reproduction in sub- fertility due to unilateral cryptorchidism. Andrologia 1996, 28, 141–144. [Google Scholar] [CrossRef]
- Vernaeve, V.; Krikilion, A.; Verheyen, G.; Van Steirteghem, A.; Devroey, P.; Tournaye, H. Outcome of testicular sperm recovery and ICSI in patients with non-obstructive azoospermia with a history of orchidopexy. Hum. Reprod. 2004, 19, 2307–2312. [Google Scholar] [CrossRef] [Green Version]
- Raman, J.D.; Schlegel, P.N. Testicular sperm extraction with intracytoplasmic sperm injection is successful for the treatment of nonobstructive azoospermia associated with cryptorchidism. J. Urol. 2003, 170 Pt 1, 1287–1290. [Google Scholar] [CrossRef]
- Wiser, A.; Raviv, G.; Weissenberg, R.; Elizur, S.; Levron, J.; Machtinger, R.; Madgar, I. Does age at orchidopexy impact on the results of testicular sperm extraction? Reprod. Biomed. Online 2009, 19, 778–783. [Google Scholar] [CrossRef] [Green Version]
- Bîcă, O.; Sârbu, I.; Ciongradi, C. Pediatric and Adolescent Oncofertility in Male Patients—From Alpha to Omega. Genes 2021, 12, 701. [Google Scholar] [CrossRef]
- Forbes, C.; Flannigan, R.; Schlegel, P.N. Spermatogonial stem cell transplantation and male infertility: Current status and future directions. Arab. J. Urol. 2018, 16, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Venditti, M.; Fasano, C.; Minucci, S.; Serino, I.; Sinisi, A.A.; Dale, B.; Di Matteo, L. DAAM1 and PREP are involved in human spermatogenesis. Reprod. Fertil. Dev. 2020, 32, 484. [Google Scholar] [CrossRef]
- Venditti, M.; Arcaniolo, D.; De Sio, M.; Minucci, S. Preliminary Investigation on the Involvement of Cytoskeleton-Related Proteins, DAAM1 and PREP, in Human Testicular Disorders. Int. J. Mol. Sci. 2021, 22, 8094. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciongradi, C.I.; Sârbu, I.; Iliescu Halițchi, C.O.; Benchia, D.; Sârbu, K. Fertility of Cryptorchid Testis—An Unsolved Mistery. Genes 2021, 12, 1894. https://doi.org/10.3390/genes12121894
Ciongradi CI, Sârbu I, Iliescu Halițchi CO, Benchia D, Sârbu K. Fertility of Cryptorchid Testis—An Unsolved Mistery. Genes. 2021; 12(12):1894. https://doi.org/10.3390/genes12121894
Chicago/Turabian StyleCiongradi, Carmen Iulia, Ioan Sârbu, Codruța Olimpiada Iliescu Halițchi, Diana Benchia, and Klara Sârbu. 2021. "Fertility of Cryptorchid Testis—An Unsolved Mistery" Genes 12, no. 12: 1894. https://doi.org/10.3390/genes12121894