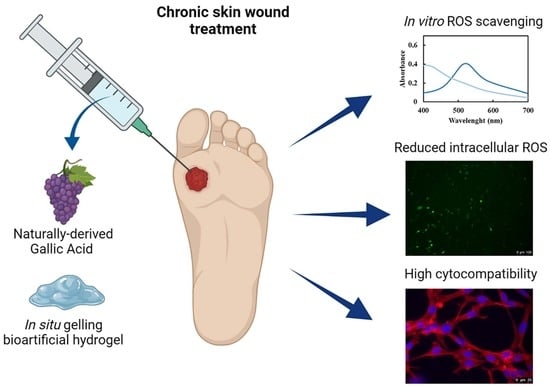
In Situ Forming Bioartificial Hydrogels with ROS Scavenging Capability Induced by Gallic Acid Release with Potential in Chronic Skin Wound Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Poly(Ether Urethane) Chemical Characterization
2.2. Bioartificial Hydrogel Properties in Physiological-Mimicking Conditions
2.2.1. Hydrogel Thermo-Responsiveness
2.2.2. Hydrogel Responsiveness to Physiological-like Fluids
2.3. In Vitro GA Release Test and Assessment of Its Scavenging Properties
2.4. In Vitro Evaluation of System Cytocompatibility
2.5. GA Protective Effects under Induced Oxidative Stress
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Poly(Ether Urethane) Synthesis and Exposure of Secondary Amino Groups
4.3. Poly(Ether Urethane) Chemical Characterization
4.4. Bioartificial Hydrogel Preparation
4.5. Qualitative Evaluation of the Thermo-Sensitivity of Macromolecular Hydrogels
4.6. Rheological Characterization of Macromolecular Hydrogels
4.7. Bioartificial Hydrogel Responsiveness to Physiological-Like Fluids
4.8. Preparation of Gallic Acid-Loaded Hydrogels
4.9. Gallic Acid Release Study
4.10. In Vitro Assessment of ROS Scavenging Effects
4.11. Preliminary Assessment of Hydrogel Cytocompatibility
4.11.1. Cytocompatibility of Hydrogel Extracts
4.11.2. Cytocompatibility of Gallic Acid
4.12. Cell Protective Effect Exerted by Released GA
4.12.1. Induction of an In Vitro ROS Microenvironment
4.12.2. Measurement of Intracellular ROS under GA Scavenging Activity
4.13. Immunofluorescence Staining to Study Morphological Changes in Fibroblast Cytoskeleton
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef]
- Clark, R.A.F. Overview and General Considerations of Wound Repair. In The Molecular and Cellular Biology of Wound Repair; Clark, R.A.F., Henson, P.M., Eds.; Springer: Boston, MA, USA, 1998. [Google Scholar] [CrossRef]
- Roy, S.; Khanna, S.; Nallu, K.; Hunt, T.K.; Sen, C.K. Dermal wound healing is subject to redox control. Mol. Ther. 2006, 13, 211–220. [Google Scholar] [CrossRef]
- D’Autreaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell. Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, 31S–38S. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, P.A.; Trump, B.F. Inflammation and oxidative stress in carcinogenesis. Cancer Cells 1991, 3, 1–7. [Google Scholar]
- Rather, H.A.; Thakore, R.; Singh, R.; Jhala, D.; Singh, S.; Vasita, R. Antioxidative study of Cerium Oxide nanoparticle functionalised PCL-Gelatin electrospun fibers for wound healing application. Bioact. Mater. 2018, 3, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, F.; Liu, W.; Liu, Y.; Guo, Z.; Xiao, C.; Zhuang, X.; Chen, X. Reactive oxide species-scavenging lipid-polymer nanoparticles for neuroprotection after spinal cord injury. Appl. Mater. Today 2021, 24, 101109. [Google Scholar] [CrossRef]
- Wu, H.; Li, F.; Wang, S.; Lu, J.; Li, J.; Du, Y.; Sun, X.; Chen, X.; Gao, J.; Ling, D. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018, 151, 66–77. [Google Scholar] [CrossRef]
- Winter, G.D. Formation of the Scab and the Rate of Epithelization of Superficial Wounds in the Skin of the Young Domestic Pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Moh, S.H.; Son, D.H.; You, S.; Kinyua, A.W.; Ko, C.M.; Song, M.; Yeo, J.; Choi, J.H.; Kim, K.W. Gallic acid promotes wound healing in normal and hyperglucidic conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef]
- Shukla, S.; Singh, B.; Singh, A.; Singh, C. Emerging and advanced drug delivery systems for improved biopharmaceutical attributes of gallic acid: A review. Phytomedicine Plus 2022, 2, 100369. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, C.; Huang, B.; Fei, P. Preparation of acylated pectin with gallic acid through enzymatic method and their emulsifying properties, antioxidation activities and antibacterial activities. Int. J. Biol. Macromol. 2020, 165, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Sagdicoglu Celep, A.G.; Demirkaya, A.; Solak, E.B. Antioxidant and anticancer activities of gallic acid loaded sodium alginate microspheres on colon cancer. Curr. Appl. Phys. 2022, 40, 30–42. [Google Scholar] [CrossRef]
- Moradi, A.; Abolfathi, M.; Javadian, M.; Heidarian, E.; Roshanmehr, H.; Khaledi, M.; Nouri, A. Gallic Acid Exerts Nephroprotective, Anti-Oxidative Stress, and Anti-Inflammatory Effects Against Diclofenac-Induced Renal Injury in Malerats. Arch. Med. Res. 2021, 52, 380–388. [Google Scholar] [CrossRef]
- Heidarian, E.; Keloushadi, M.; Ghatreh-Samani, K.; Valipour, P. The reduction of IL-6 gene expression, pAKT, pERK1/2, pSTAT3 signaling pathways and invasion activity by gallic acid in prostate cancer PC3 cells. Biomed. Pharmacother. 2016, 84, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Y.; Jia, R.; Fang, F.; Liu, Y.; Cui, W. Computational and biological investigation of the soybean lecithin–gallic acid complex for ameliorating alcoholic liver disease in mice with iron overload. Food Funct. 2019, 10, 5203–5214. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, T.; Wu, C.; Wang, J.; Ye, F.; Cui, M.; Sun, S.; Zhang, Y.; Li, Y.; Dong, Z. A Shape-Adaptive Gallic Acid Driven Multifunctional Adhesive Hydrogel Loaded with Scolopin2 for Wound Repair. Pharmaceuticals 2022, 15, 1422. [Google Scholar] [CrossRef]
- Nguyen-Ngo, C.; Salomon, C.; Lai, A.; Willcox, J.C.; Lappas, M. Anti-inflammatory effects of gallic acid in human gestational tissues in vitro. Reproduction 2020, 160, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Giordani, B.; Basnet, P.; Mishchenko, E.; Luppi, B.; Škalko-Basnet, N. Utilizing liposomal quercetin and gallic acid in localized treatment of vaginal candida infections. Pharmaceutics 2020, 12, 9. [Google Scholar] [CrossRef]
- Radwan, S.A.; El-Maadawy, W.H.; Yousry, C.; ElMeshad, A.N.; Shoukri, R.A. Zein/phospholipid composite nanoparticles for successful delivery of gallic acid into ahscs: Influence of size, surface charge, and vitamin a coupling. Int. J. Nanomed. 2020, 15, 7995–8018. [Google Scholar] [CrossRef] [PubMed]
- Persano, F.; Gigli, G.; Leporatti, S. Lipid-polymer hybrid nanoparticles in cancer therapy: Current overview and future directions. Nano Express 2021, 2, 012006. [Google Scholar] [CrossRef]
- Ahmed, H.H.; Galal, A.F.; Shalby, A.B.; Abd-Rabou, A.A.; Mehaya, F.M. Improving anti-cancer potentiality and bioavailability of gallic acid by designing polymeric nanocomposite formulation. Asian Pac. J. Canc. Prevent. 2018, 19, 3137–3146. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, A.; Sumnu, G.; Sahin, S. Fabrication of gallic acid loaded Hydroxypropyl methylcellulose nanofibers by electrospinning technique as active packaging material. Carbohydr. Polym. 2019, 208, 241–250. [Google Scholar] [CrossRef]
- Zhao, Z.; Lu, M.; Mao, Z.; Xiao, J.; Huang, Q.; Lin, X.; Cao, Y. Modulation of interfacial phenolic antioxidant distribution in Pickering emulsions via interactions between zein nanoparticles and gallic acid. Int. J. Biol. Macromol. 2020, 152, 223–233. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.S.; et al. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.; Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Abdou, E.M.; Masoud, M.M. Gallic acid–PAMAM and gallic acid–phospholipid conjugates, physicochemical characterization and in vivo evaluation. Pharm. Dev. Technol. 2018, 23, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Laurano, R.; Chiono, V.; Ceresa, C.; Fracchia, L.; Zoso, A.; Ciardelli, G.; Boffito, M. Custom-design of intrinsically antimicrobial polyurethane hydrogels as multifunctional injectable delivery systems for mini-invasive wound treatment. Eng. Regen. 2021, 2, 263–278. [Google Scholar] [CrossRef]
- Ciccone, V.; Zazzetta, M.; Morbidelli, L. Comparison of the Effect of Two Hyaluronic Acid Preparations on Fibroblast and Endothelial Cell Functions Related to Angiogenesis. Cells 2019, 8, 1479. [Google Scholar] [CrossRef] [PubMed]
- Bot, P.T.; Hoefer, I.E.; Piek, J.J.; Pasterkamp, G. Hyaluronic Acid: Targeting Immune Modulatory Components of the Extracellular Matrix in Atherosclerosis. Curr. Med. Chem. 2008, 15, 786–791. [Google Scholar] [CrossRef]
- Bot, P.T.; Pasterkamp, G.; Goumans, M.J.; Strijder, C.; Moll, F.L.; De Vries, J.P.; Pals, S.T.; De Kleijn, D.P.; Piek, J.J.; Hoefer, I.E. Hyaluronic acid metabolism is increased in unstable plaques. Eur. J. Clin. Investig. 2010, 40, 818–827. [Google Scholar] [CrossRef]
- Schmidt, J.; Pilbauerova, N.; Soukup, T.; Suchankova-Kleplova, T.; Suchanek, J. Low Molecular Weight Hyaluronic Acid Effect on Dental Pulp Stem Cells In Vitro. Biomolecules 2021, 11, 22. [Google Scholar] [CrossRef]
- Valachová, K.; El Meligy, M.A.; Šoltés, L. Hyaluronic acid and chitosan-based electrospun wound dressings: Problems and solutions. Int. J. Biol. Macromol. 2022, 206, 74–91. [Google Scholar] [CrossRef]
- Polaka, S.; Katare, P.; Pawar, B.; Vasdev, N.; Gupta, T.; Rajpoot, K.; Sengupta, P.; Tekade, R.K. Emerging ROS-Modulating Technologies for Augmentation of the Wound Healing Process. ACS Omega 2022, 7, 30657–30672. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Kaya, G.; Tran, C.; Sorg, O.; Hotz, R.; Grand, D.; Carraux, P.; Didierjean, L.; Stamenkovic, I.; Saurat, J.H. Hyaluronate fragments reverse skin atrophy by a CD44-dependent mechanism. PLoS Med. 2006, 3, e493. [Google Scholar] [CrossRef]
- Slevin, M.; Kumar, S.; Gaffney, J. Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways affecting vascular endothelial cell mitogenic and wound healing responses. J. Biol. Chem. 2002, 277, 41046–41059. [Google Scholar] [CrossRef] [PubMed]
- Laurano, R.; Abrami, M.; Grassi, M.; Ciardelli, G.; Boffito, M.; Chiono, V. Using Poloxamer® 407 as Building Block of Amphiphilic Poly(ether urethane)s: Effect of its Molecular Weight Distribution on Thermo-Sensitive Hydrogel Performances in the Perspective of Their Biomedical Application. Front. Mater. 2020, 7, 594515. [Google Scholar] [CrossRef]
- Laurano, R.; Cassino, C.; Ciardelli, G.; Chiono, V.; Boffito, M. Polyurethane-based thiomers: A new multifunctional copolymer platform for biomedical applications. React. Funct. Polym. 2020, 146, 104413. [Google Scholar] [CrossRef]
- Laurano, R.; Boffito, M.; Cassino, C.; Liberti, F.; Ciardelli, G.; Chiono, V. Design of Injectable Bioartificial Hydrogels by Green Chemistry for Mini-Invasive Applications in the Biomedical or Aesthetic Medicine Fields. Gels 2023, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Eelkema, R.; Pich, A. Pros and Cons: Supramolecular or Macromolecular: What Is Best for Functional Hydrogels with Advanced Properties? Adv. Mater. 2020, 32, e1906012. [Google Scholar] [CrossRef] [PubMed]
- Laurano, R.; Boffito, M.; Torchio, A.; Cassino, C.; Chiono, V.; Ciardelli, G. Plasma Treatment of Polymer Powder as an Effective Tool to Functionalize Polymers: Case Study Application on an Amphiphilic Polyurethane. Polymers 2019, 11, 2109. [Google Scholar] [CrossRef] [PubMed]
- Torchio, A.; Cassino, C.; Lavella, M.; Gallina, A.; Stefani, A.; Boffito, M.; Ciardelli, G. Injectable supramolecular hydrogels based on custom-made poly(ether urethane)s and α-cyclodextrins as efficient delivery vehicles of curcumin. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112194. [Google Scholar] [CrossRef]
- Sadeghi, R.; Jahani, F. Salting-In and Salting-Out of Water-Soluble Polymers in Aqueous Salt Solutions. J. Phys. Chem. B 2012, 116, 5234–5241. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Aswal, V.K. Improved Micellar Hydration and Gelation Characteristics of PEO−PPO−PEO Triblock Copolymer Solutions in the Presence of LiCl. J. Phys. Chem. B 2008, 112, 7726–7731. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P.; Holzwarth, J.F. Differential Scanning Calorimetry Investigation of the Effect of Salts on Aqueous Solution Properties of an Amphiphilic Block Copolymer (Poloxamer). Langmuir 1997, 13, 6074–6082. [Google Scholar] [CrossRef]
- Cutting, K.F. Wound exudate: Composition and functions. Br. J. Community Nurs. 2003, 8, S4–S9. [Google Scholar] [CrossRef]
- Lei, Y.; Ouyang, H.; Peng, W.; Yu, X.; Jin, L.; Li, S. Effect of NaCl on the Rheological, Structural, and Gelling Properties of Walnut Protein Isolate-κ-Carrageenan Composite Gels. Gels 2022, 8, 259. [Google Scholar] [CrossRef]
- Zhou, F.F.; Pan, M.K.; Liu, Y.; Guo, N.; Zhang, Q.; Wang, J.H. Effects of Na+ on the cold gelation between a low-methoxyl pectin extracted from Premna microphylla turcz and soy protein isolate. Food Hydrocoll. 2020, 104, 105762. [Google Scholar] [CrossRef]
- Laurano, R.; Boffito, M.; Abrami, M.; Grassi, M.; Zoso, A.; Chiono, V.; Ciardelli, G. Dual stimuli-responsive polyurethane-based hydrogels as smart drug delivery carriers for the advanced treatment of chronic skin wounds. Bioact. Mater. 2021, 6, 3013–3024. [Google Scholar] [CrossRef]
- Laurano, R.; Boffito, M. Thermosensitive Micellar Hydrogels as Vehicles to Deliver Drugs with Different Wettability. Front. Bioeng. Biotechnol. 2020, 8, 708. [Google Scholar] [CrossRef]
- Op ‘t Veld, R.C.; Walboomers, X.F.; Jansen, J.A.; Wagener, F.A.D.T.G. Design Considerations for Hydrogel Wound Dressings: Strategic and Molecular Advances. Tissue Eng. Part. B Rev. 2020, 26, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jun, C.D.; Suk, K.; Choi, B.J.; Lim, H.; Park, S.; Lee, S.H.; Shin, H.Y.; Kim, D.K.; Shin, T.Y. Gallic Acid Inhibits Histamine Release and Pro-inflammatory Cytokine Production in Mast Cells. Toxicol. Scie. 2006, 91, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 2007, 30, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, O.; Taiwo, F.O.; Femi, O.; Ajayi, O.; Oziegbe, M.; Kelani, M.; Adewole, A. In vitro Antimicrobial and Antioxidant Analysis of Gallic Acid from the Leaves of Ludwigia abyssinica A. Rich. Eur. J. Med. Plants 2014, 4, 1098–1112. [Google Scholar] [CrossRef]
- Voinchet, V.; Vasseur, P.; Kern, J. Efficacy and safety of hyaluronic acid in the management of acute wounds. Am. J. Clin. Dermatol. 2006, 7, 353–357. [Google Scholar] [CrossRef]
- Kawano, Y.; Patrulea, V.; Sublet, E.; Borchard, G.; Iyoda, T.; Kageyama, R.; Morita, A.; Seino, S.; Yoshida, H.; Jordan, O.; et al. Wound Healing Promotion by Hyaluronic Acid: Effect of Molecular Weight on Gene Expression and In Vivo Wound Closure. Pharmaceuticals 2021, 14, 301. [Google Scholar] [CrossRef]
- Martin, C.; Aibani, N.; Callan, J.F.; Callan, B. Recent advances in amphiphilic polymers for simultaneous delivery of hydrophobic and hydrophilic drugs. Ther. Deliv. 2015, 7, 15–31. [Google Scholar] [CrossRef]
- Thi, P.L.; Lee, Y.; Tran, D.L.; Thi, T.T.H.; Kang, J.I.; Park, K.M.; Park, K.D. In situ forming and reactive oxygen species-scavenging gelatin hydrogels for enhancing wound healing efficacy. Acta Biomater. 2020, 103, 142–152. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Mo, h.; Ma, H.; Yuan, E.; Li, Q. Characterization and DPPH Radical Scavenging Activity of Gallic Acid-Lecithin Complex. Trop. J. Pharm. Res. 2014, 13, 1333–1338. [Google Scholar] [CrossRef]
- Hao, T.; Li, J.; Yao, F.; Dong, D.; Wang, Y.; Yang, B.; Wang, C. Injectable Fullerenol/Alginate Hydrogel for Suppression of Oxidative Stress Damage in Brown Adipose-Derived Stem Cells and Cardiac Repair. ACS Nano 2017, 11, 5474–5488. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html/ (accessed on 5 August 2023).
- Khorsandi, K.; Hosseinzadeh, R.; Esfahani, H.; Zandsalimi, K.; Shahidi, F.K.; Abrahamse, H. Accelerating skin regeneration and wound healing by controlled ROS from photodynamic treatment. Inflamm. Regener. 2022, 42, 40–60. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Hanna, P.M.; Mason, R.P. The Origin of the Hydroxyl Radical Oxygen in the Fenton Reaction. Free. Radic. Biol. Med. 1997, 22, 885–888. [Google Scholar] [CrossRef]
- Rajavel, K.; Gomathi, R.; Manian, S.; Kumar, R.T.R. Characterization of tannic acid- and gallic acid-functionalized single- and multiwalled carbon nanotubes and an in vitro evaluation of their antioxidant properties. J. Taibah Univ. Med. Sci. 2016, 11, 469–477. [Google Scholar] [CrossRef]
- Zhu, Y.; Matsumura, Y.; Velayutham, M.; Foley, L.M.; Hitchens, T.K.; Wagner, W.R. Reactive oxygen species scavenging with a biodegradable, thermally responsive hydrogel compatible with soft tissue injection. Biomaterials 2018, 177, 98–112. [Google Scholar] [CrossRef]












| HA (% w/v) | D-DHP407 (% w/v) | Solvent | |
|---|---|---|---|
| HA/D-DHP407_5%_ddH2O | 5.0% | 5.0% | ddH2O |
| HA/D-DHP407_6%_ddH2O | 6.0% | 6.0% | ddH2O |
| HA/D-DHP407_7.5%_ddH2O | 7.5% | 7.5% | ddH2O |
| HA/D-DHP407_5%_DPBS | 5.0% | 5.0% | DPBS |
| HA/D-DHP407_6%_DPBS | 6.0% | 6.0% | DPBS |
| HA/D-DHP407_7.5%_DPBS | 7.5% | 7.5% | DPBS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurano, R.; Torchio, A.; Ciardelli, G.; Boffito, M. In Situ Forming Bioartificial Hydrogels with ROS Scavenging Capability Induced by Gallic Acid Release with Potential in Chronic Skin Wound Treatment. Gels 2023, 9, 731. https://doi.org/10.3390/gels9090731
Laurano R, Torchio A, Ciardelli G, Boffito M. In Situ Forming Bioartificial Hydrogels with ROS Scavenging Capability Induced by Gallic Acid Release with Potential in Chronic Skin Wound Treatment. Gels. 2023; 9(9):731. https://doi.org/10.3390/gels9090731
Chicago/Turabian StyleLaurano, Rossella, Alessandro Torchio, Gianluca Ciardelli, and Monica Boffito. 2023. "In Situ Forming Bioartificial Hydrogels with ROS Scavenging Capability Induced by Gallic Acid Release with Potential in Chronic Skin Wound Treatment" Gels 9, no. 9: 731. https://doi.org/10.3390/gels9090731