Holographic Recording of Unslanted Volume Transmission Gratings in Acrylamide/Propargyl Acrylate Hydrogel Layers: Towards Nucleic Acids Biosensing
Abstract
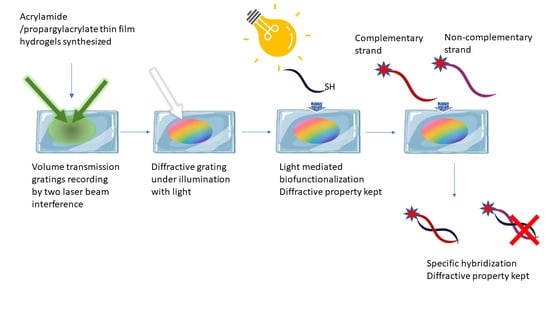
:1. Introduction
2. Results and Discussions
Biofunctionalization of VTGs and Hybridization Assays by Fluorescence Detection
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Hydrogel Layers Preparation
4.3. Morphology Characterization
4.4. Swelling Behavior Studies
4.5. Incubation Step before Recording
4.6. Holographic Recording and Probe Set-Up
4.7. Holographic Recording and Characterization of Hydrogel Layers
4.8. Biofuncionalization of VTGs and Hybridization/Dehybridization Experiments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yetisen, A.K.; Naydenova, I.; Da Cruz Vasconcellos, F.; Blyth, J.; Lowe, C.R. Holographic Sensors: Three-Dimensional Analyte-Sensitive Nanostructures and Their Applications. Chem. Rev. 2014, 114, 10654–10696. [Google Scholar] [CrossRef] [PubMed]
- Blanche, P.A. Holographic Recording Media and Devices. Encycl. Mod. Opt. 2018, 4, 87–101. [Google Scholar] [CrossRef]
- Mihaylova, E.M. Water-Soluble Holographic Photopolymers for a Sustainable Future—A Review. Coatings 2022, 12, 1765. [Google Scholar] [CrossRef]
- Pal, A.K.; Labella, E.; Goddard, N.J.; Gupta, R. Photofunctionalizable Hydrogel for Fabricating Volume Optical Diffractive Sensors. Macromol. Chem. Phys. 2019, 220, 1900228. [Google Scholar] [CrossRef]
- Sun, X.; Agate, S.; Salem, K.S.; Lucia, L.; Pal, L. Hydrogel-Based Sensor Networks: Compositions, Properties, and Applications—A Review. ACS Appl. Bio Mater. 2021, 4, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic Hydrogels: Synthesis, Novel Trends, and Applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Ding, M.; Fan, X.; Hu, J.; Chen, Y.; Li, J.; Li, Z.; Liu, W. A 4arm-PEG Macromolecule Crosslinked Chitosan Hydrogels as Antibacterial Wound Dressing. Carbohydr. Polym. 2022, 277, 118871. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Fan, X.; Hu, J.; Li, J.; Rong, J.; Wang, W.; Chen, Y.; Liu, W.; Chen, J.; Chen, Y. Construction and Function of Robust and Moist Bilayer Chitosan-Based Hydrogel Wound Dressing. Mater. Des. 2023, 226, 111604. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Allonas, X.; Burget, D. Photopolymerization Reactions under Visible Lights: Principle, Mechanisms and Examples of Applications. Prog. Org. Coat. 2003, 47, 16–36. [Google Scholar] [CrossRef]
- Neipp, C.; Taleb, S.I.; Francés, J.; Fernández, R.; Puerto, D.; Calzado, E.M.; Gallego, S.; Beléndez, A. Analysis of the Imaging Characteristics of Holographic Waveguides Recorded in Photopolymers. Polymers 2020, 12, 1485. [Google Scholar] [CrossRef]
- Ramírez, M.G.; Lucío, M.I.; Morales-Vidal, M.; Beléndez, A.; Bañuls, M.J.; Maquieira, Á.; Pascual, I. Holographic Transmission Gratings Stored in a Hydrogel Matrix. Photosensit. Mater. Their Appl. 2020, 11367, 22–29. [Google Scholar] [CrossRef]
- Berramdane, K.; Ramírez, M.G.; Zezza, P.; Lucío, M.I.; Bañuls, M.J.; Maquieira, Á.; Morales-Vidal, M.; Beléndez, A.; Pascual, I. Processing of Holographic Hydrogels in Liquid Media: A Study by High-Performance Liquid Chromatography and Diffraction Efficiency. Polymers 2022, 14, 2089. [Google Scholar] [CrossRef]
- Jiang, N.; Davies, S.; Jiao, Y.; Blyth, J.; Butt, H.; Monteelongo, Y.; Yetisen, A.K. Doubly Photopolymerized Holographic Sensors. ACS Sens. 2021, 6, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.R.; Sheridan, J.T. A Review of the Modelling of Free-Radical Photopolymerization in the Formation of Holographic Gratings. J. Opt. A Pure Appl. Opt. 2009, 11, 024008. [Google Scholar] [CrossRef]
- Davies, S.; Hu, Y.; Jiang, N.; Blyth, J.; Kaminska, M.; Liu, Y.; Yetisen, A.K. Holographic Sensors in Biotechnology. Adv. Funct. Mater. 2021, 31, 2105645. [Google Scholar] [CrossRef]
- Marshall, A.J.; Young, D.S.; Blyth, J.; Kabilan, S.; Lowe, C.R. Metabolite-Sensitive Holographic Biosensors. Anal. Chem. 2004, 76, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Lalisse, A.; Mohtar, A.A.; Nguyen, M.C.; Carminati, R.; Plain, J.; Tessier, G. Quantitative Temperature Measurements in Gold Nanorods Using Digital Holography. ACS Appl. Mater. Interfaces 2021, 13, 10313–10320. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, D.; Zhou, K.; Mao, D.; Liu, L.; Wang, H.; Wang, W.; Song, Q. Temperature-induced spectrum response of volume grating as an effective strategy for holographic sensing in acrylamide polymer part I: Sensing. Appl. Opt. 2016, 55, 9907–9916. [Google Scholar] [CrossRef]
- Bianco, G.; Ferrara, M.A.; Borbone, F.; Zuppardi, F.; Roviello, A.; Striano, V.; Coppola, G. Volume Holographic Gratings as Optical Sensor for Heavy Metal in Bathing Waters. Opt. Sens. 2015, 9506, 95062B. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Qasim, M.M.; Nosheen, S.; Wilkinson, T.D.; Lowe, C.R. Pulsed Laser Writing of Holographic Nanosensors. J. Mater. Chem. C 2014, 2, 3569–3576. [Google Scholar] [CrossRef]
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Wearable Contact Lens Biosensors for Continuous Glucose Monitoring Using Smartphones. ACS Nano 2018, 12, 5452–5462. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.; Hu, Y.; Blyth, J.; Jiang, N.; Yetisen, A.K. Reusable Dual-Photopolymerized Holographic Glucose Sensors. Adv. Funct. Mater. 2023, 33, 2214197. [Google Scholar] [CrossRef]
- Zezza, P.; Lucío, M.I.; Fernández, E.; Maquieira, Á.; Bañuls, M.J. Surface Micro-Patterned Biofunctionalized Hydrogel for Direct Nucleic Acid Hybridization Detection. Biosensors 2023, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Kogelnik, H. Coupled Wave Theory for Thick Hologram Gratings. Bell Syst. Tech. J. 1969, 48, 2909–2947. [Google Scholar] [CrossRef]
- Toal, V. Introduction to Holography, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]










| Incubation Time (Days) | Layer Thickness (µm) | Recording Beams Intensity (mW/cm2) | Recording Exposure Time (s) | Maximum DE Achieved (%) |
|---|---|---|---|---|
| 1 | 300 | 7.5 | 100 | 40 |
| 2 | 300 | 7.5 | 100 | 80 |
| 3 | 300 | 7.5 | 100 | 50 |
| VTG Thickness (µm) | Refractive Index Modulation (RIM) | |
|---|---|---|
| After recording | 190 | 0.0010 |
| First washing | 60 | 0.0015 |
| Second washing | 62 | 0.0017 |
| Incubation Solution | AM (g) | MBA (g) | TEA (mL) | EB (mL) |
|---|---|---|---|---|
| A | 1 | 0.2 | 1 | 4 |
| B | 2 | 0.4 | 1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zezza, P.; Lucío, M.I.; Naydenova, I.; Bañuls, M.-J.; Maquieira, Á. Holographic Recording of Unslanted Volume Transmission Gratings in Acrylamide/Propargyl Acrylate Hydrogel Layers: Towards Nucleic Acids Biosensing. Gels 2023, 9, 710. https://doi.org/10.3390/gels9090710
Zezza P, Lucío MI, Naydenova I, Bañuls M-J, Maquieira Á. Holographic Recording of Unslanted Volume Transmission Gratings in Acrylamide/Propargyl Acrylate Hydrogel Layers: Towards Nucleic Acids Biosensing. Gels. 2023; 9(9):710. https://doi.org/10.3390/gels9090710
Chicago/Turabian StyleZezza, Paola, María Isabel Lucío, Izabela Naydenova, María-José Bañuls, and Ángel Maquieira. 2023. "Holographic Recording of Unslanted Volume Transmission Gratings in Acrylamide/Propargyl Acrylate Hydrogel Layers: Towards Nucleic Acids Biosensing" Gels 9, no. 9: 710. https://doi.org/10.3390/gels9090710