Carboxymethyl Chitosan/Sodium Alginate/Chitosan Quaternary Ammonium Salt Composite Hydrogel Supported 3J for the Treatment of Oral Ulcer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Compound 3J
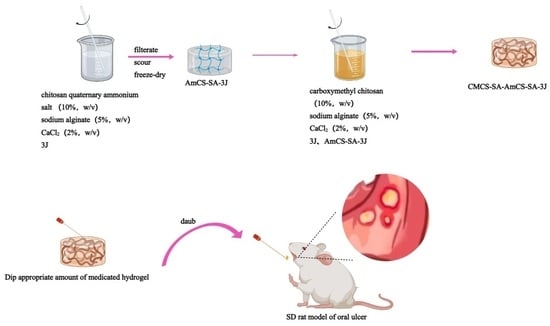
2.2. Hydrogel Preparation, Characterization, and Drug Release
2.2.1. Hydrogel Preparation and Characterization
2.2.2. Drug Release from Hydrogels
2.3. In Vivo Antibacterial Properties
2.4. Anti-Inflammatory Effects of Hydrogels and 3J
2.5. In Vivo Efficacy Assay
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Compound 3J
4.3. Preparation of Composite Hydrogels
4.3.1. Preparation of AmCS-SA-3J
4.3.2. Synthesis of Complex Hydrogels
4.4. Characterization of Composite Hydrogels
4.4.1. FT-IR
4.4.2. Surface Topography Analysis
4.4.3. Determination of Swelling, Water Retention, and Degradation
4.4.4. Test of Mechanical Properties of Hydrogels
4.4.5. Evaluation of Entrapment Efficiency (EE) and Drug Loading (DL)
4.5. In Vitro Release Test
4.6. In Vitro Anti-Bacterial Assay
4.7. In Vitro Anti-Inflammatory Assay
4.8. Evaluation of Oral Ulcer Animal Model
4.9. H&E Staining
4.10. Experimental Data Statistics and Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edgar, N.R.; Saleh, D.; Miller, R. Recurrent Aphthous Stomatitis: A Review. J. Clin. Aesthetic Dermatol. 2017, 10, 26–36. [Google Scholar]
- Chiang, C.P.; Yu-Fong Chang, J.; Wang, Y.P.; Wu, Y.H.; Wu, Y.C.; Sun, A. Recurrent aphthous stomatitis—Etiology, serum autoanti-bodies, anemia, hematinic deficiencies, and management. J. Formos. Med. Assoc. 2019, 118, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.M. The basics of mouth sores. J. Am. Dent. Assoc. 2022, 153, 1014. [Google Scholar] [CrossRef] [PubMed]
- Nalbantoğlu, B.; Nalbantoğlu, A. Vitamin D Levels in Children with Recurrent Aphthous Stomatitis. Ear Nose Throat J. 2020, 99, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Öztekin, A.; Öztekin, C. Vitamin D levels in patients with recurrent aphthous stomatitis. BMC Oral. Health 2018, 18, 186. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A.J.; Dabade, T.S.; Burkemper, N.M. Diagnosing oral ulcers. JAAPA 2015, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saikaly, S.K.; Saikaly, T.S.; Saikaly, L.E. Recurrent aphthous ulceration: A review of potential causes and novel treatments. J. Dermatol. Treat. 2018, 29, 542–552. [Google Scholar] [CrossRef]
- Gasmi Benahmed, A.; Noor, S.; Menzel, A.; Gasmi, A. Oral Aphthous: Pathophysiology, Clinical Aspects and Medical Treatment. Arch. Razi Inst. 2021, 76, 1155–1163. [Google Scholar] [PubMed]
- Zhang, Z.; Zhang, Q.; Gao, S.; Xu, H.; Guo, J.; Yan, F. Antibacterial, anti-inflammatory and wet-adhesive poly(ionic liquid)-based oral patch for the treatment of oral ulcers with bacterial infection. Acta Biomater. 2023, 166, 254–265. [Google Scholar] [CrossRef]
- Zhang, W.; Bao, B.; Jiang, F.; Zhang, Y.; Zhou, R.; Lu, Y.; Lin, S.; Lin, Q.; Jiang, X.; Zhu, L. Promoting Oral Mucosal Wound Healing with a Hydrogel Adhesive Based on a Phototriggered S-Nitrosylation Coupling Reaction. Adv. Mater. 2021, 33, e2105667. [Google Scholar] [CrossRef]
- Geng, Y.; Xue, H.; Zhang, Z.; Panayi, A.C.; Knoedler, S.; Zhou, W.; Mi, B.; Liu, G. Recent advances in carboxymethyl chitosan-based materials for biomedical applications. Carbohydr. Polym. 2023, 305, 120555. [Google Scholar] [CrossRef]
- Chang, G.; Dang, Q.; Liu, C.; Wang, X.; Song, H.; Gao, H.; Sun, H.; Zhang, B.; Cha, D. Carboxymethyl chitosan and carboxymethyl cellulose based self-healing hydrogel for accelerating diabetic wound healing. Carbohydr. Polym. 2022, 292, 119687. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Long, T.; Wan, Y.; Li, B.; Xu, Z.; Zhao, L.; Mu, C.; Ge, L.; Li, D. Dual-drug loaded polysaccharide-based self-healing hydrogels with multifunctionality for promoting diabetic wound healing. Carbohydr. Polym. 2023, 312, 120824. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, S.S.; Ehsani, M.; Zandi, M.; Saeed, M.; Sabeti, M. Polysaccharide-based (kappa carrageenan/carboxymethyl chitosan) nan-ofibrous membrane loaded with antifibrinolytic drug for rapid hemostasis- in vitro and in vivo evaluation. Int. J. Biol. Macromol. 2023, 247, 125786. [Google Scholar] [CrossRef] [PubMed]
- Jungprasertchai, N.; Chuysinuan, P.; Ekabutr, P.; Niamlang, P.; X Supaphol, P. Freeze-Dried Car-boxymethyl Chitosan/Starch Foam for Use as a Haemostatic Wound Dressing. J. Polym. Environment. 2021, 30, 1106–1117. [Google Scholar] [CrossRef]
- Pan, Q.; Zhou, C.; Yang, Z.; He, Z.; Wang, C.; Liu, Y.; Song, S.; Gu, H.; Hong, K.; Yu, L.; et al. Preparation and characterization of chitosan derivatives modified with quaternary ammonium salt and quaternary phosphate salt and its effect on tropical fruit preservation. Food Chem. 2022, 387, 132878. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xin, M.; Li, M.; Liu, W.; Mao, Y. Effect of the structure of chitosan quaternary phosphonium salt and chitosan quaternary ammonium salt on the antibacterial and antibiofilm activity. Int. J. Biol. Macromol. 2023, 242, 124877. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Poverenov, E. Hydrophilic Chitosan Derivatives: Synthesis and Applications. Chemistry 2022, 28, e202202156. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Li, Q.; Liu, X.; Guo, Z. Preparation of Cross-linked Chitosan Quaternary Ammonium Salt Hydrogel Films Loading Drug of Gentamicin Sulfate for Antibacterial Wound Dressing. Mar. Drugs 2021, 19, 479. [Google Scholar] [CrossRef]
- Makarova, A.O.; Derkach, S.R.; Khair, T.; Kazantseva, M.A.; Zuev, Y.F.; Zueva, O.S. Ion-Induced Polysaccharide Gelation: Peculiarities of Alginate Egg-Box Association with Different Divalent Cations. Polymers 2023, 15, 1243. [Google Scholar] [CrossRef] [PubMed]
- Samp, M.A.; Iovanac, N.C.; Nolte, A.J. Sodium Alginate Toughening of Gelatin Hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 3176–3182. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Xu, K.; Huang, Y.; Liu, S.; Wang, T.; Wang, W.; Hu, W.; Liu, L.; Xing, M.; Yang, S. Viscosity and degradation controlled injectable hydrogel for esophageal endoscopic submucosal dissection. Bioact. Mater. 2020, 6, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-known Polymer. J. Pharm. Sci. 2022, 111, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhou, J.; An, Y.; Li, M.; Zhang, J.; Yang, S. Modification, 3D printing process and application of sodium alginate based hy-drogels in soft tissue engineering: A review. Int. J. Biol. Macromol. 2023, 232, 123450. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yi, W.; Zhang, Y.; Wu, H.; Fan, H.; Zhao, J.; Wang, S. Sodium alginate hydrogel containing platelet-rich plasma for wound healing. Colloids Surf. B Biointerfaces 2023, 222, 113096. [Google Scholar] [CrossRef]
- Xie, M.; Zeng, Y.; Wu, H.; Wang, S.; Zhao, J. Multifunctional carboxymethyl chitosan/oxidized dextran/sodium alginate hydrogels as dressing for hemostasis and closure of infected wounds. Int. J. Biol. Macromol. 2022, 219, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Hecht, H.; Srebnick, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Hertel, L.W.; Boder, G.B.; Kroin, J.S.; Rinzel, S.M.; A Poore, G.; Todd, G.C.; Grindey, G.B. Evaluation of the antitumor activity of gemcitabine (2′,2′-difluoro-2′-deoxycytidine). Cancer Res. 1990, 50, 4417–4422. [Google Scholar]
- Han, C.; Salyer, A.E.; Kim, E.H.; Jiang, X.; Jarrard, R.E.; Powers, M.S.; Kirchhoff, A.M.; Salvador, T.K.; Chester, J.A.; Hockerman, G.H.; et al. Evaluation of difluoromethyl ketones as agonists of the γ-aminobutyric acid type B (GABAB) receptor. J. Med. Chem. 2013, 56, 2456–2465. [Google Scholar] [CrossRef]
- Chen, C.; Lu, D.; Sun, T.; Zhang, T. JAK3 inhibitors for the treatment of inflammatory and autoimmune diseases: A patent review (2016–present). Expert. Opin. Ther. Pat. 2022, 32, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.J.; Xu, J.Y.; Cui, X.L.; Qu, J.; Sun, W.Q.; Hu, J.H.; Zhao, S.W.; Chen, W.-H.; Li, H.L.; Wu, J.Q. Synthesis of difluoromethyl carbinols from the Friedel–Crafts reaction of electron-rich arenes with difluorovinyl arylsulfonates. Org. Chem. Front. 2022, 9, 6273–6280. [Google Scholar] [CrossRef]
- Himiniuc, L.M.; Socolov, R.; Nica, I.; Agop, M.; Volovat, C.; Ochiuz, L.; Vasincu, D.; Rotundu, A.M.; Rosu, I.A.; Ghizdovat, V.; et al. Theoretical and Experimental Aspects of Sodium Diclofenac Salt Release from Chitosan-Based Hydrogels and Possible Applications. Gels 2023, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Ghazwani, M.; Hani, U.; Alam, A.; Alqarni, M.H. Quality-by-Design-Assisted Optimization of Carvacrol Oil-Loaded Niosomal Gel for Anti-Inflammatory Efficacy by Topical Route. Gels 2023, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Mouro, C.; Gomes, A.P.; Gouveia, I.C. Emulsion Electrospinning of PLLA/PVA/Chitosan with Hypericum perforatum L. as an Antibact. Nanofibrous Wound Dressing. Gels 2023, 9, 353. [Google Scholar] [PubMed]
- Min, Z.; Yang, L.; Hu, Y.; Huang, R. Oral microbiota dysbiosis accelerates the development and onset of mucositis and oral ulcers. Front. Microbiol. 2023, 14, 1061032. [Google Scholar] [CrossRef] [PubMed]
- Terai, H.; Ueno, T.; Suwa, Y.; Omori, M.; Yamamoto, K.; Kasuya, S. Candida is a protractive factor of chronic oral ulcers among usual outpatients. Jpn. Dent. Sci. Rev. 2018, 54, 52–58. [Google Scholar] [CrossRef]
- Laheij, A.M.; de Soet, J.J. Can Oral Microflora Affect Oral Ulcerative Mucositis? Curr. Opin. Support. Palliat. Care. 2014, 8, 180. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Freitas, M.O.; Fonseca, A.P.R.; de Aguiar, M.T.; Dias, C.C.; Avelar, R.L.; Sousa, F.B.; Alves, A.P.N.N.; Silva, P.G.d.B. Tumor necrosis factor alpha (TNF-α) blockage reduces acute inflammation and delayed wound healing in oral ulcer of rats. Inflammopharmacology 2022, 30, 1781–1798. [Google Scholar] [CrossRef]
- Surboyo, M.D.C.; Boedi, R.M.; Hariyani, N.; Santosh, A.B.R.; Manuaba, I.B.P.P.; Cecilia, P.H.; Ambarawati, I.G.A.D.; Parmadiati, A.E.; Ernawati, D.S. The expression of TNF-α in recurrent aphthous stomatitis: A systematic review and meta-analysis. Cytokine 2022, 157, 155946. [Google Scholar] [CrossRef] [PubMed]
- Karakus, N.; Yigit, S.; Rustemoglu, A.; Kalkan, G.; Bozkurt, N. Effects of interleukin (IL)-6 gene polymorphisms on recurrent aphthous stomatitis. Arch. Dermatol. Res. 2013, 306, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Zhang, J.-Q. Association between interleukin gene polymorphisms and risk of recurrent oral ulceration. Genet. Mol. Res. 2015, 14, 6838–6843. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Xiao, J.; Guan, S.; Geng, Z.; Zhao, R.; Gao, B. A hydrogen-bonded antibacterial curdlan-tannic acid hydrogel with an an-tioxidant and hemostatic function for wound healing. Carbohydr. Polym. 2022, 285, 119235. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Long, L.; Yang, L.; Fu, D.; Hu, C.; Kong, Q.; Wang, Y. Inflammation-Responsive Drug-Loaded Hydrogels with Sequential Hemostasis, Antibacterial, and Anti-Inflammatory Behavior for Chronically Infected Diabetic Wound Treatment. ACS Appl. Mater. Interfaces 2021, 13, 33584–33599. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Khan, A.; Wang, T.; Song, Q.; Han, C.; Wang, Q.; Gao, L.; Huang, X.; Li, P.; Huang, W. Mussel-Inspired Hydrogel with Potent in Vivo Contact-Active Antimicrobial and Wound Healing Promoting Activities. ACS Appl. Bio Mater. 2019, 2, 3329–3340. [Google Scholar] [CrossRef]
- Chen, P.; Yao, H.; Su, W.; He, Y.; Cheng, K.; Wang, Y.; Peng, W.; Li, P. Sleep deprivation worsened oral ulcers and delayed healing process in an experimental rat model. Life Sci. 2019, 232, 116594. [Google Scholar] [CrossRef]
- Kimura, H.; Nagoshi, T.; Oi, Y.; Yoshii, A.; Tanaka, Y.; Takahashi, H.; Kashiwagi, Y.; Tanaka, T.D.; Yoshimura, M. Treatment with atrial natriuretic peptide induces adipose tissue browning and exerts thermogenic actions in vivo. Sci. Rep. 2021, 11, 17466. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.; Chen, D.; Geng, Y.; Li, J.; Ou, Y.; Zeng, Z.; Yin, C.; Qian, X.; Qiu, X.; Li, G.; et al. Carboxymethyl Chitosan/Sodium Alginate/Chitosan Quaternary Ammonium Salt Composite Hydrogel Supported 3J for the Treatment of Oral Ulcer. Gels 2023, 9, 659. https://doi.org/10.3390/gels9080659
Lin T, Chen D, Geng Y, Li J, Ou Y, Zeng Z, Yin C, Qian X, Qiu X, Li G, et al. Carboxymethyl Chitosan/Sodium Alginate/Chitosan Quaternary Ammonium Salt Composite Hydrogel Supported 3J for the Treatment of Oral Ulcer. Gels. 2023; 9(8):659. https://doi.org/10.3390/gels9080659
Chicago/Turabian StyleLin, Tao, Dandan Chen, Yan Geng, Jiayu Li, Yanghui Ou, Zhijun Zeng, Canqiang Yin, Xudong Qian, Xiang Qiu, Gang Li, and et al. 2023. "Carboxymethyl Chitosan/Sodium Alginate/Chitosan Quaternary Ammonium Salt Composite Hydrogel Supported 3J for the Treatment of Oral Ulcer" Gels 9, no. 8: 659. https://doi.org/10.3390/gels9080659