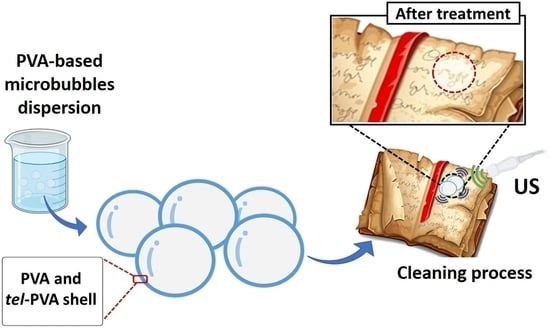
Ultrasound-Stimulated PVA Microbubbles as a Green and Handy Tool for the Cleaning of Cellulose-Based Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cleaning of Modern Paper Samples
2.2. Cleaning of Ancient Paper Samples
3. Conclusions
4. Materials and Methods
4.1. Reagents and Materials
4.2. Synthesis and Cleaning Procedures
4.2.1. PVAMBs Synthesis and Characterization
4.2.2. PVA Hydrogel Synthesis and Characterization
4.2.3. Protocol Application
4.3. Techniques
4.3.1. ATR-FTIR Spectroscopy
4.3.2. X-ray Diffraction (XRD) Analysis
4.3.3. Tensile Tests
4.3.4. High-Performance Liquid Chromatography (HPLC) Analysis and pH Measurements
4.3.5. Colorimetry
4.3.6. Optical Microscopy
4.3.7. Dynamic Light Scattering (DLS) Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Proniewicz, L.M.; Paluszkiewicz, C.; Wesełucha-Birczyńska, A.; Majcherczyk, H.; Barański, A.; Konieczna, A. FT-IR and FT-Raman Study of Hydrothermally Degradated Cellulose. J. Mol. Struct. 2001, 596, 163–169. [Google Scholar] [CrossRef]
- Chiriu, D.; Ricci, P.C.; Cappellini, G.; Carbonaro, C.M. Ancient and Modern Paper: Study on Ageing and Degradation Process by Means of Portable NIR μ-Raman Spectroscopy. Microchem. J. 2018, 138, 26–34. [Google Scholar] [CrossRef]
- Łojewska, J.; Lubańska, A.; Miśkowiec, P.; Łojewski, T.; Proniewicz, L.M. FTIR in Situ Transmission Studies on the Kinetics of Paper Degradation via Hydrolytic and Oxidative Reaction Paths. Appl. Phys. A 2006, 83, 597–603. [Google Scholar] [CrossRef]
- Potthast, A.; Ahn, K.; Becker, M.; Eichinger, T.; Kostic, M.; Böhmdorfer, S.; Jeong, M.J.; Rosenau, T. Acetylation of Cellulose—Another Pathway of Natural Cellulose Aging during Library Storage of Books and Papers. Carbohydr. Polym. 2022, 287, 119323. [Google Scholar] [CrossRef] [PubMed]
- Mosca Conte, A.; Pulci, O.; Knapik, A.; Bagniuk, J.; Del Sole, R.; Lojewska, J.; Missori, M. Role of Cellulose Oxidation in the Yellowing of Ancient Paper. Phys. Rev. Lett. 2012, 108, 158301. [Google Scholar] [CrossRef]
- Liu, Y.; Fearn, T.; Strlič, M. Factorial Experimentation on Photodegradation of Historical Paper by Polychromatic Visible Radiation. Herit. Sci. 2021, 9, 130. [Google Scholar] [CrossRef]
- Zidan, Y.; El-Shafei, A.; Noshy, W.; Salim, E. A Comparative Study to Evaluate Conventional And Nonconventional Cleaning Treatments Of Cellulosic Paper Supports. Mediterr. Archaeol. Archaeom. 2017, 17, 337–353. [Google Scholar] [CrossRef]
- Moropoulou, A.; Zervos, S. The Immediate Impact of Aqueous Treatments on the Strength of Paper. Restaurator 2003, 24, 160–177. [Google Scholar] [CrossRef]
- Bicchieri, M.; Monti, M.; Piantanida, G.; Pinzari, F.; Iannuccelli, S.; Sotgiu, S.; Tireni, L. The Indian Drawings of the Poet Cesare Pascarella: Non-Destructive Analyses and Conservation Treatments. Anal. Bioanal. Chem. 2012, 402, 1517–1528. [Google Scholar] [CrossRef]
- Isca, C.; Fuster-López, L.; Yusá-Marco, D.J.; Casoli, A. An Evaluation of Changes Induced by Wet Cleaning Treatments in the Mechanical Properties of Paper Artworks. Cellulose 2015, 22, 3047–3062. [Google Scholar] [CrossRef]
- Casini, A.; Chelazzi, D.; Baglioni, P. Advanced Methodologies for the Cleaning of Works of Art. Sci. China Technol. Sci. 2023, 1–21. [Google Scholar] [CrossRef]
- Mazzuca, C.; Micheli, L.; Marini, F.; Bevilacqua, M.; Bocchinfuso, G.; Palleschi, G.; Palleschi, A. Rheoreversible Hydrogels in Paper Restoration Processes: A Versatile Tool. Chem. Cent. J. 2014, 8, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Severini, L.; Titubante, M.; Gong, D.; Micheli, L.; Mazzuca, C.; Gong, Y. Gellan Gum Hydrogel as an Aqueous Treatment Method for Xuan Paper. Restaurator. Int. J. Preserv. Libr. Arch. Mater. 2021, 42, 37–54. [Google Scholar] [CrossRef]
- Mazzuca, C.; Micheli, L.; Lettieri, R.; Cervelli, E.; Coviello, T.; Cencetti, C.; Sotgiu, S.; Iannuccelli, S.; Palleschi, G.; Palleschi, A. How to Tune a Paper Cleaning Process by Means of Modified Gellan Hydrogels. Microchem. J. 2016, 126, 359–367. [Google Scholar] [CrossRef]
- Mazzuca, C.; Poggi, G.; Bonelli, N.; Micheli, L.; Baglioni, P.; Palleschi, A. Innovative Chemical Gels Meet Enzymes: A Smart Combination for Cleaning Paper Artworks. J. Colloid Interface Sci. 2017, 502, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Librando, V.; Minniti, Z.; Lorusso, S. Caratterizzazione Di Carta Antica e Moderna a Mezzo Spettroscopia FTIR e Micro-Raman Ancient and Modern Paper Characterization by FTIR and Micro-Raman Spectroscopy. Conserv. Sci. Cult. Herit. 2012, 11, 249–268. [Google Scholar] [CrossRef]
- Baglioni, M.; Poggi, G.; Chelazzi, D.; Baglioni, P. Advanced Materials in Cultural Heritage Conservation. Molecules 2021, 26, 3967. [Google Scholar] [CrossRef]
- Collings, T.; Milner, D. The Nature and Identification of Cotton Paper Making Fibres in Paper. Pap. Conserv. 1984, 8, 59–71. [Google Scholar] [CrossRef]
- Čabalová, I.; Kačík, F.; Gojný, J.; Češek, B.; Milichovský, M.; Mikala, O.; Tribulová, T.; Ďurkovič, J. Changes in the Chemical and Physical Properties of Paper Documents Due to Natural Ageing. BioResources 2017, 12, 2618–2634. [Google Scholar] [CrossRef] [Green Version]
- Calvini, P.; Gorassini, A. FTIR—Deconvolution Spectra of Paper Documents. Restaurator 2002, 23, 48–66. [Google Scholar] [CrossRef]
- Mills, J.S.; White, R. National Gallery Technical Bulletin; The National Gallery Company Limited: London, UK, 1980; pp. 65–67. [Google Scholar]
- Gorassini, A.; Adami, G.; Calvini, P.; Giacomello, A. ATR-FTIR Characterization of Old Pressure Sensitive Adhesive Tapes in Historic Papers. J. Cult. Herit. 2016, 21, 775–785. [Google Scholar] [CrossRef]
- Micheli, L.; Mazzuca, C.; Missori, M.; Teodonio, L.; Mosca Conte, A.; Pulci, O.; Arcadipane, L.; Dominijanni, S.; Palleschi, A.; Palleschi, G.; et al. Interdisciplinary Approach to Develop a Disposable Real Time Monitoring Tool for the Cleaning of Graphic Artworks. Application on “Le Nozze Di Psiche”. Microchem. J. 2018, 138, 369–378. [Google Scholar] [CrossRef]
- Duncan, T.T.; Vicenzi, E.P.; Brogdon-Grantham, S.A. Imaging Rough Paper to Evaluate Methods for Soot Removal. Micros. Today 2022, 30, 30–33. [Google Scholar] [CrossRef]
- Di Napoli, B.; Franco, S.; Severini, L.; Tumiati, M.; Buratti, E.; Titubante, M.; Nigro, V.; Gnan, N.; Micheli, L.; Ruzicka, B.; et al. Gellan Gum Microgels as Effective Agents for a Rapid Cleaning of Paper. ACS Appl. Polym. Mater. 2020, 2, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.T.; Vicenzi, E.P.; Lam, T.; Brogdon-Grantham, S.A. A Comparison of Materials for Dry Surface Cleaning Soot-Coated Papers of Varying Roughness: Assessing Efficacy, Physical Surface Changes, and Residue. J. Am. Inst. Conserv. 2023, 62, 152–167. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Ponassi, V.; Martorana, V. Tailoring of Physical and Chemical Properties of Macro- and Microhydrogels Based on Telechelic PVA. Biomacromolecules 2002, 3, 1255–1262. [Google Scholar] [CrossRef] [Green Version]
- Mazzuca, C.; Severini, L.; Missori, M.; Tumiati, M.; Domenici, F.; Micheli, L.; Titubante, M.; Bragaglia, M.; Nanni, F.; Paradossi, G.; et al. Evaluating the Influence of Paper Characteristics on the Efficacy of New Poly(Vinyl Alcohol) Based Hydrogels for Cleaning Modern and Ancient Paper. Microchem. J. 2020, 155, 104716. [Google Scholar] [CrossRef]
- Mazzuca, C.; Severini, L.; Domenici, F.; Toumia, Y.; Mazzotta, F.; Micheli, L.; Titubante, M.; Di Napoli, B.; Paradossi, G.; Palleschi, A. Polyvinyl Alcohol Based Hydrogels as New Tunable Materials for Application in the Cultural Heritage Field. Colloids Surf. B Biointerfaces 2020, 188, 110777. [Google Scholar] [CrossRef]
- Da Ros, V.; Oddo, L.; Toumia, Y.; Guida, E.; Minosse, S.; Strigari, L.; Strolin, S.; Paolani, G.; Di Giuliano, F.; Floris, R.; et al. PVA-Microbubbles as a Radioembolization Platform: Formulation and the In Vitro Proof of Concept. Pharmaceutics 2023, 15, 217. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Severini, L.; Domenici, F.; Dabagov, S.; Guglielmotti, V.; Hampai, D.; Micheli, L.; Placidi, E.; Titubante, M.; Mazzuca, C.; et al. Ultrasound-Stimulated PVA Microbubbles for Adhesive Removal from Cellulose-Based Materials: A Groundbreaking Low-Impact Methodology. ACS Appl. Mater. Interfaces 2021, 13, 24207–24217. [Google Scholar] [CrossRef]
- Paradossi, G.; Grossman, R.; Riccitelli, F.; Todaro, F.; Ram, Z.; Schioppa, S.; Domenici, F. Toward a Theranostic Device for Gliomas. Biochem. Biophys. Res. Commun. 2023, 671, 124–131. [Google Scholar] [CrossRef]
- Pan, C.; Industry, L.; Tan, W.; Kuang, B.; Fang, Y.; Lin, Y.; Cai, X. A Novel Glue Remover for Pressure Sensitive Tapes on Aged Paper. Wood Res. 2019, 64, 759–772. [Google Scholar]
- Tavagnacco, L.; Chiessi, E.; Severini, L.; Franco, S.; Buratti, E.; Capocefalo, A.; Brasili, F.; Mosca Conte, A.; Missori, M.; Angelini, R.; et al. Molecular Origin of the Two-Step Mechanism of Gellan Aggregation. Sci. Adv. 2023, 9, eadg4392. [Google Scholar] [CrossRef] [PubMed]
- Domenici, F.; Brasili, F.; Oddo, L.; Cerroni, B.; Bedini, A.; Bordi, F.; Paradossi, G. Long-Term Physical Evolution of an Elastomeric Ultrasound Contrast Microbubble. J. Colloid Interface Sci. 2019, 540, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, M.; Hepher, M.J. Effects of Ultrasound Energy on Degradation of Cellulose Material. Ultrason. Sonochemistry 2000, 7, 265–268. [Google Scholar] [CrossRef]
- Li, W.; Yue, J.; Liu, S. Preparation of Nanocrystalline Cellulose via Ultrasound and Its Reinforcement Capability for Poly(Vinyl Alcohol) Composites. Ultrason. Sonochemistry 2012, 19, 479–485. [Google Scholar] [CrossRef]
- Grishenkov, D.; Pecorari, C.; Brismar, T.B.; Paradossi, G. Characterization of Acoustic Properties of PVA-Shelled Ultrasound Contrast Agents: Ultrasound-Induced Fracture (Part II). Ultrasound Med. Biol. 2009, 35, 1139–1147. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose Crystallinity Index: Measurement Techniques and Their Impact on Interpreting Cellulase Performance. Biotechnol Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Margutti, S.; Conio, G.; Calvini, P.; Pedemonte, E. Hydrolytic and Oxidative Degradation of Paper. Restaurator 2001, 22, 67–83. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and Infrared Spectroscopy of Carbohydrates: A Review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Łojewska, J.; Missori, M.; Lubańska, A.; Grimaldi, P.; Ziȩba, K.; Proniewicz, L.M.; Congiu Castellano, A. Carbonyl Groups Development on Degraded Cellulose. Correlation between Spectroscopic and Chemical Results. Appl. Phys. A 2007, 89, 883–887. [Google Scholar] [CrossRef]
- Tzvetkov, G.; Paradossi, G.; Tortora, M.; Fernandes, P.; Fery, A.; Graf-Zeiler, B.; Fink, R.H. Water-Dispersible PVA-Based Dry Microballoons with Potential for Biomedical Applications. Mater. Sci. Eng. C 2010, 30, 412–416. [Google Scholar] [CrossRef]
- D’Orsi, R.; Canale, V.C.; Cancelliere, R.; Hassan Omar, O.; Mazzuca, C.; Micheli, L.; Operamolla, A. Tailoring the Chemical Structure of Cellulose Nanocrystals by Amine Functionalization. Eur J Org Chem 2023, 26. [Google Scholar] [CrossRef]
- Kataoka, Y.; Kondo, T. FT-IR Microscopic Analysis of Changing Cellulose Crystalline Structure during Wood Cell Wall Formation. Macromolecules 1998, 31, 760–764. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR Spectroscopy Characterization of Poly (Vinyl Alcohol) Hydrogel with Different Hydrolysis Degree and Chemically Crosslinked with Glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Łojewska, J.; Miśkowiec, P.; Łojewski, T.; Proniewicz, L.M. Cellulose Oxidative and Hydrolytic Degradation: In Situ FTIR Approach. Polym. Degrad. Stab. 2005, 88, 512–520. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Severini, L.; D’Andrea, A.; Redi, M.; Dabagov, S.B.; Guglielmotti, V.; Hampai, D.; Micheli, L.; Cancelliere, R.; Domenici, F.; Mazzuca, C.; et al. Ultrasound-Stimulated PVA Microbubbles as a Green and Handy Tool for the Cleaning of Cellulose-Based Materials. Gels 2023, 9, 509. https://doi.org/10.3390/gels9070509
Severini L, D’Andrea A, Redi M, Dabagov SB, Guglielmotti V, Hampai D, Micheli L, Cancelliere R, Domenici F, Mazzuca C, et al. Ultrasound-Stimulated PVA Microbubbles as a Green and Handy Tool for the Cleaning of Cellulose-Based Materials. Gels. 2023; 9(7):509. https://doi.org/10.3390/gels9070509
Chicago/Turabian StyleSeverini, Leonardo, Alessia D’Andrea, Martina Redi, Sultan B. Dabagov, Valeria Guglielmotti, Dariush Hampai, Laura Micheli, Rocco Cancelliere, Fabio Domenici, Claudia Mazzuca, and et al. 2023. "Ultrasound-Stimulated PVA Microbubbles as a Green and Handy Tool for the Cleaning of Cellulose-Based Materials" Gels 9, no. 7: 509. https://doi.org/10.3390/gels9070509