Progress in Surface Modification of Titanium Implants by Hydrogel Coatings
Abstract
:1. Introduction
2. Classification of Hydrogel Coatings
2.1. Natural Hydrogel Coating
2.1.1. Collagen-Based Hydrogel Coating
2.1.2. Gelatin-Based Hydrogel Coating
2.1.3. Chitosan-Based Hydrogel Coating
2.1.4. Alginate-Based Hydrogel Coating
2.2. Synthetic Hydrogel Coatings
3. Binding Method of Hydrogel Coating and Titanium Implant (Preparation Method of Hydrogel Coating)
3.1. Electrochemical Methods
3.2. Sol–Gel Method
3.3. Layer-by-Layer Self-Assembly
4. Characterization Methods of Surface Modification
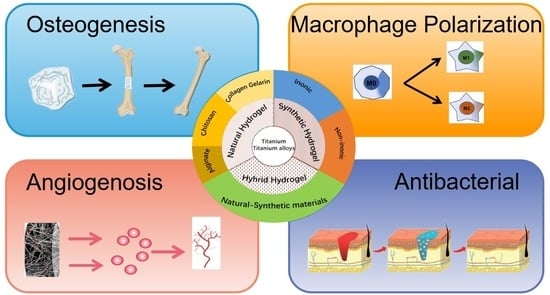
5. Application of the Hydrogel Coating
5.1. Osseointegration
5.2. Angiogenesis
5.3. Macrophage Polarization
5.4. Antibacterial
5.5. Drug Delivery
6. Conclusions and Future Protects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bothe, R.T.; Beaton, L.E.; Davenport, H.A. Reaction of bone to multiple metallic implants. Surg. Gynecol. Obstet. 1940, 71, 598–602. [Google Scholar]
- Bordbar-Khiabani, A.; Gasik, M. Electrochemical and biological characterization of Ti-Nb-Zr-Si alloy for orthopedic applications. Sci. Rep. 2023, 13, 2312. [Google Scholar] [CrossRef] [PubMed]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Nayan, N.H.M. An updated review on surface functionalisation of titanium and its alloys for implants applications. Mater. Today Proc. 2021, 42, 270–282. [Google Scholar] [CrossRef]
- Del Castillo, R.; Chochlidakis, K.; Galindo-Moreno, P.; Ercoli, C. Titanium Nitride Coated Implant Abutments: From Technical Aspects and Soft tissue Biocompatibility to Clinical Applications. A Literature Review. J. Prosthodont. 2022, 31, 571–578. [Google Scholar] [CrossRef]
- Prabhakar, V.; Chidambaranathan, A.S.; Balasubramanium, M. Effect of Cathodic Arc Plasma Deposition on Shear Bond Strength between Palladium Cobalt Chromium Coated with Titanium Nitride and Titanium Aluminium Nitride with Ceramic. Contemp. Clin. Dent. 2021, 12, 49–54. [Google Scholar] [PubMed]
- Zhang, R.; Wan, Y.; Ai, X.; Zhang, D. Corrosion resistance and biological activity of TiO2 implant coatings produced in oxygen-rich environments. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; You, L.; Wang, T.; Li, B. Enhanced Osseointegration of Titanium Implants by Surface Modification with Silicon-doped Titania Nanotubes. Int. J. Nanomed. 2020, 15, 8583–8594. [Google Scholar] [CrossRef]
- Shimabukuro, M. Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Alshimaysawee, S.; Fadhel Obaid, R.; Al-Gazally, M.E.; Bathaei, M.S. Recent Advancements in Metallic Drug-Eluting Implants. Pharmaceutics 2023, 15, 223. [Google Scholar] [CrossRef]
- Yuan, Y.X.; Luo, R.D.; Ren, J.K.; He, Z.Y. Design of a new Ti-Mo-Cu alloy with excellent mechanical and antibacterial properties as implant materials. Mater. Lett. 2022, 306, 130875. [Google Scholar] [CrossRef]
- Lupi, S.M.; Torchia, M.; Rizzo, S. Biochemical Modification of Titanium Oral Implants: Evidence from in Vivo Studies. Materials 2021, 14, 2798. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.C.; Mao, L.L.; Shi, Y.T.; Hu, Y.H. Biocompatibility of Ti-6Al-4V titanium alloy implants with laser microgrooved surfaces. Mater. Technol. 2022, 37, 2039–2048. [Google Scholar] [CrossRef]
- Bohara, S.; Suthakorn, J. Surface coating of orthopedic implant to enhance the osseointegration and reduction of bacterial colonization: A review. Biomater. Res. 2022, 26, 26. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, E.; Cometa, S.; Cioffi, N.; Sabbatini, L. Analytical investigations of poly (acrylic acid) coatings electrodeposited on titanium-based implants: A versatile approach to biocompatibility enhancement. Anal. Bioanal. Chem. 2007, 389, 2055–2063. [Google Scholar] [CrossRef]
- Nagai, M.; Hayakawa, T.; Fukatsu, A.; Kato, T. In vitro study of collagen coating of titanium implants for initial cell attachment. Dent. Mater. J. 2002, 21, 250–260. [Google Scholar] [CrossRef]
- Hauser, J.; Ring, A.; Schaffran, A.; Langer, S. In vivo analysis of tissue response to plasma-treated collagen-I-coated titanium alloys. Eur. Surg. Res. 2009, 43, 262–268. [Google Scholar] [CrossRef]
- Raita, Y.; Komatsu, K.; Hayakawa, T. Pilot study of gingival connective tissue responses to 3-dimensional collagen nanofiber-coated dental implants. Dent. Mater. J. 2015, 34, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Vanderleyden, E.; Van Bael, S.; Chai, Y.C.; Dubruel, P. Gelatin functionalised porous titanium alloy implants for orthopaedic applications. Mat. Sci. Eng. C Mater. 2014, 42, 396–404. [Google Scholar] [CrossRef]
- Mahin, T.; Torab, A.; Negahdari, R.; Sharifi, S. The Antibacterial Effects of Healing Abutments Coated with Gelatin-curcumin Nanocomposite. Pharm. Nanotechnol. 2023. [Google Scholar] [CrossRef]
- López-Valverde, N.; Aragoneses, J.; López-Valverde, A.; Aragoneses, J.M. Role of chitosan in titanium coatings. trends and new generations of coatings. Front. Bioeng. Biotechnol. 2022, 10, 907589. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Chesnutt, B.M.; Yuan, Y.; Ong, J.L. The integration of chitosan-coated titanium in bone: An in vivo study in rabbits. Implant 2007, 16, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.X.; Ao, H.Y.; Wang, L.; Tang, T.T. Quaternised chitosan coating on titanium provides a self-protective surface that prevents bacterial colonisation and implant-associated infections. RSC Adv. 2015, 5, 54304–54311. [Google Scholar] [CrossRef]
- Jian, Y.H.; Yang, C.; Zhang, J.X.; Du, Y.M. One-step electrodeposition of Janus chitosan coating for metallic implants with anti-corrosion properties. Colloid Surf. 2022, 641, 128498. [Google Scholar] [CrossRef]
- Guo, C.C.; Cui, W.D.; Wang, X.W.; Chen, J.L. Poly-L-lysine/Sodium Alginate Coating Loading Nanosilver for Improving the Antibacterial Effect and Inducing Mineralization of Dental Implants. ACS Omega 2020, 5, 10562–10571. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Jia, L.; Geng, Z.; Liu, Y. The Incorporation of Strontium in a Sodium Alginate Coating on Titanium Surfaces for Improved Biological Properties. Biomed. Res. Int. 2017, 2017, 9867819. [Google Scholar] [CrossRef] [PubMed]
- Abdul Azam, F.A.; Ismail, H.; Abdul Hamid, M.A. Characterizations on Morphology and Adhesion of Calcium Silicate Coating on Ti6Al4V Substrate. Key Eng. Mater. 2016, 694, 83–87. [Google Scholar] [CrossRef]
- Sille, I.E.; Pissinis, D.E.; Fagali, N.S.; Schilardi, P.L. Antimicrobial-Loaded Polyacrylamide Hydrogels Supported on Titanium as Reservoir for Local Drug Delivery. Pathogens 2023, 12, 202. [Google Scholar] [CrossRef]
- Buxadera-Palomero, J.; Calvo, C.; Torrent-Camarero, S.; Rodríguez, D. Biofunctional polyethylene glycol coatings on titanium: An in vitro-based comparison of functionalization methods. Colloid Surf. 2017, 152, 367–375. [Google Scholar] [CrossRef]
- Xiao, D.Q.; Liu, Q.; Weng, J. Room-temperature attachment of PLGA microspheres to titanium surfaces for implant-based drug release. Appl. Surf. Sci. 2014, 30, 112–118. [Google Scholar] [CrossRef]
- Iafiscol, M.; Quirici, N.; Foltran, I.; Rimondini, L. Electrospun collagen mimicking the reconstituted extracellular matrix improves osteoblastic differentiation onto titanium surfaces. J. Nanosci. Nanotechnol. 2013, 13, 4720–4726. [Google Scholar] [CrossRef]
- Becker, D.; Geissler, U.; Hempel, U.; Bierbaum, S.; Scharnweber, D.; Worch, H.; Wenzel, K.W. Proliferation and differentiation of rat calvarial osteoblasts on type I collagen-coated titanium alloy. J. Biomed. Mater. Res. 2002, 59, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Bierbaum, S.; Hempel, U.; Geissler UHanke, T.; Scharnweber, D.; Wenzel, K.W.; Worch, H. Modification of Ti6Al4V surfaces using collagen i, iii, and fibronectin. Ii. Influence on osteoblast responses. J. Biomed. Mater. Res. A 2003, 67, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Geissler, U.; Hempel, U.; Wolf, C.; Scharnweber, D.; Worch, H.; Wenzel, K. Collagen type I-coating of Ti6Al4V promotes adhesion of osteoblasts. J. Biomed. Mater. Res. 2000, 51, 752–760. [Google Scholar] [CrossRef]
- Ritz, U.; Nusselt, T.; Sewing, A.; Hofmann, A. The effect of different collagen modifications for titanium and titanium nitrite surfaces on functions of gingival fibroblasts. Clin. Oral. Investig. 2017, 21, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Sharan, J.; Koul, V.; Dinda, A.K.; Singh, M.P. Bio-functionalization of grade V titanium alloy with type I human collagen for enhancing and promoting human periodontal fibroblast cell adhesion—An in-vitro study. Colloid Surf. 2018, 161, 1–9. [Google Scholar] [CrossRef]
- Morra, M.; Cassinelli, C.; Meda, L.; Giardino, R. Surface analysis and effects on interfacial bone microhardness of collagen-coated titanium implants: A rabbit model. Int. J. Oral Maxillofac. Implant. 2005, 20, 23–30. [Google Scholar]
- Ciobanu, G.; Ciobanu, O. Investigation on the effect of collagen and vitamins on biomimetic hydroxyapatite coating formation on titanium surfaces. Mat. Sci. Eng. C Mater. 2013, 33, 1683–1688. [Google Scholar] [CrossRef]
- Ruiz, G.C.M.; Cruz, M.A.E.; Faria, A.N.; Ramos, A.P. Biomimetic collagen/phospholipid coatings improve formation of hydroxyapatite nanoparticles on titanium. Mat. Sci. Eng. C Mater. 2017, 77, 102–110. [Google Scholar] [CrossRef]
- Patty, D.J.; Nugraheni, A.D.; Dewi Ana, I.; Yusuf, Y. Mechanical Characteristics and Bioactivity of Nanocomposite Hydroxyapatite/Collagen Coated Titanium for Bone Tissue Engineering. Bioengineering 2022, 9, 784. [Google Scholar] [CrossRef]
- Pokorný, M.; Suchý, T.; Kotzianová, A.; Čejka, Z. Surface Treatment of Acetabular Cups with a Direct Deposition of a Composite Nanostructured Layer Using a High Electrostatic Field. Molecules 2020, 25, 1173. [Google Scholar] [CrossRef]
- Iwanami-Kadowaki, K.; Uchikoshi, T.; Uezono, M.; Moriyama, K. Development of novel bone-like nanocomposite coating of hydroxyapatite/collagen on titanium by modified electrophoretic deposition. J. Biomed. Mater. Res. A 2021, 109, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bai, L.; Zhang, Y.; Hang, R. Type I collagen decorated nanoporous network on titanium implant surface promotes osseointegration through mediating immunomodulation, angiogenesis, and osteogenesis. Biomaterials 2022, 288, 121684. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Weng, L.; Li, J.; Lin, J. Regulation of Macrophage Polarization by Mineralized Collagen Coating to Accelerate the Osteogenic Differentiation of Mesenchymal Stem Cells. ACS Biomater. Sci. Eng. 2022, 8, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Bai, H.; Huang, L. Sustained Release of VEGF to Promote Angiogenesis and Osteointegration of Three-Dimensional Printed Biomimetic Titanium Alloy Implants. Front. Bioeng. Biotechnol. 2021, 9, 757767. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.N.; Hou, S.; Park, S.; Jeong, K.J. Gelatin Hydrogel Combined with Polydopamine Coating to Enhance Tissue Integration of Medical Implants. ACS Biomater. Sci. Eng. 2018, 4, 3471–3477. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, Z.; Wang, X.; Jia, R. Construction and osteogenic effects of 3D-printed porous titanium alloy loaded with VEGF/BMP-2 shell-core microspheres in a sustained-release system. Front. Bioeng. Biotechnol. 2022, 10, 1028278. [Google Scholar] [CrossRef] [PubMed]
- Van den Bulcke, A.I.; Bogdanov, B.; de Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methaerylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Cheng, H.; Yue, K.; Kazemzadeh-Narbat, M.; Khademhosseini, A. Mussel-Inspired Multifunctional Hydrogel Coating for Prevention of Infections and Enhanced Osteogenesis. ACS Appl. Mater. Interfaces 2017, 9, 11428–11439. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Choi, A.J.; Park, J.E.; Lee, M.H. Antibacterial Activity and Biocompatibility with the Concentration of Ginger Fraction in Biodegradable Gelatin Methacryloyl (GelMA) Hydrogel Coating for Medical Implants. Polymers 2022, 14, 5317. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Leng, J.; Li, K.; Cai, K. A multifunctional hydrogel coating to direct fibroblast activation and infected wound healing via simultaneously controllable photobiomodulation and photodynamic therapies. Biomaterials 2021, 278, 121164. [Google Scholar] [CrossRef]
- Ding, Y.; Ma, R.; Liu, G.; Cai, K. Fabrication of a New Hyaluronic Acid/Gelatin Nanocomposite Hydrogel Coating on Titanium-Based Implants for Treating Biofilm Infection and Excessive Inflammatory Response. ACS Appl. Mater. Interfaces 2023, 15, 13783–13801. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cui, X.; Lindberg, G.C.J.; Woodfield, T.B.F. Hybrid fabrication of photo-clickable vascular hydrogels with additive manufactured titanium implants for enhanced osseointegration and vascularized bone formation. Biofabrication 2022, 14, 034103. [Google Scholar] [CrossRef]
- Taokaew, S.; Kaewkong, W.; Kriangkrai, W. Recent Development of Functional Chitosan-Based Hydrogels for Pharmaceutical and Biomedical Applications. Gels 2023, 9, 277. [Google Scholar] [CrossRef]
- Stevanović, M.; Đošić, M.; Janković, A.; Mišković-Stanković, V. Gentamicin-Loaded Bioactive Hydroxyapatite/Chitosan Composite Coating Electrodeposited on Titanium. ACS Biomater. Sci. Eng. 2018, 4, 3994–4007. [Google Scholar] [CrossRef] [PubMed]
- Zarghami, V.; Ghorbani, M.; Bagheri, K.P.; Shokrgozar, M.A. Improving bactericidal performance of implant composite coatings by synergism between Melittin and tetracycline. J. Mater. Sci. Mater. Med. 2022, 33, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Mortezagholi, B.; Hadizadeh, A.; Chaiyasut, C. Ciprofloxacin-Loaded Titanium Nanotubes Coated with Chitosan: A Promising Formulation with Sustained Release and Enhanced Antibacterial Properties. Pharmaceutics 2022, 14, 1359. [Google Scholar] [CrossRef] [PubMed]
- Divakar, D.D.; Jastaniyah, N.T.; Altamimi, H.G.; Haleem, S. Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens. Int. J. Biol. Macromol. 2018, 108, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Pawłowski, Ł.; Wawrzyniak, J.; Banach-Kopeć, A.; Zieliński, A. Antibacterial properties of laser-encapsulated titanium oxide nanotubes decorated with nanosilver and covered with chitosan/Eudragit polymers. Biomater. Adv. 2022, 138, 212950. [Google Scholar] [CrossRef]
- Zhang, T.; Qin, X.; Gao, Y.; Yin, P. Functional chitosan gel coating enhances antimicrobial properties and osteogenesis of titanium alloy under persistent chronic inflammation. Front. Bioeng. Biotechnol. 2023, 11, 1118487. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Hassani Besheli, N.; Deng, D.; Yang, F. Tailoring Copper-Doped Bioactive Glass/Chitosan Coatings with Angiogenic and Antibacterial Properties. Tissue Eng. Part C Methods 2022, 28, 314–324. [Google Scholar] [CrossRef]
- Stevanović, M.; Djošić, M.; Janković, A.; Mišković-Stanković, V. Antibacterial graphene-based hydroxyapatite/chitosan coating with gentamicin for potential applications in bone tissue engineering. J. Biomed. Mater. Res. A 2020, 108, 2175–2189. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, X.; Tan, L.; Wu, S. Photo-responsive chitosan/Ag/MoS2 for rapid bacteria-killing. J. Hazard. Mater. 2020, 383, 121122. [Google Scholar] [CrossRef]
- Bose, S.; Surendhiran, D.; Chun, B.S.; Kang, H.W. Facile synthesis of black phosphorus-zinc oxide nanohybrids for antibacterial coating of titanium surface. Colloid Surf. 2022, 219, 112807. [Google Scholar] [CrossRef]
- Chai, M.; An, M.; Zhang, X. Construction of a TiO2/MoSe2/CHI coating on dental implants for combating Streptococcus mutans infection. Mat. Sci. Eng. C Mater. 2021, 129, 112416. [Google Scholar] [CrossRef]
- Chen, W.; Xie, G.; Lu, Y.; Bao, J. An improved osseointegration of metal implants by pitavastatin loaded multilayer films with osteogenic and angiogenic properties. Biomaterials 2022, 280, 121260. [Google Scholar] [CrossRef]
- Wang, B.; Chen, L.; Xie, J.; Yang, L. Coating Polyelectrolyte Multilayers Loaded with Quercetin on Titanium Surfaces by Layer-By-Layer Assembly Technique to Improve Surface Osteogenesis Under Osteoporotic Condition. J. Biomed. Nanotechnol. 2021, 17, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Takanche, J.S.; Kim, J.E.; Jang, S.; Yi, H.K. Insulin growth factor binding protein-3 enhances dental implant osseointegration against methylglyoxal-induced bone deterioration in a rat model. J. Periodontal. Implant Sci. 2022, 52, 155–169. [Google Scholar] [CrossRef]
- Tao, B.; Deng, Y.; Song, L.; Cai, K. BMP2-loaded titania nanotubes coating with pH-responsive multilayers for bacterial infections inhibition and osteogenic activity improvement. Colloid Surf. 2019, 177, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, X.; Mao, M.; Sun, H. Chitosan/hydroxyapatite composite coatings on porous Ti6Al4V titanium implants: In vitro and in vivo studies. J. Periodontal. Implant Sci. 2020, 50, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, M.S.; Yakout, S.M.; Barakat, Y.F.; Sharmoukh, W. Electrophoretic deposition of hydroxyapatite/chitosan nanocomposites: The effect of dispersing agents on the coating properties. RSC Adv. 2022, 12, 27564–27581. [Google Scholar] [CrossRef]
- Rastegari, S.; Salahinejad, E. Surface modification of Ti-6Al-4V alloy for osseointegration by alkaline treatment and chitosan-matrix glass-reinforced nanocomposite coating. Carbohyd. Polym. 2019, 205, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Cai, X.; Zhou, Y.; Jiang, T. In Vitro and In Vivo Evaluation of Tetracycline Loaded Chitosan-Gelatin Nanosphere Coatings for Titanium Surface Functionalization. Macromol. Biosci. 2017, 17, 201600130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Dong, H.; Niu, Y.; Zhang, Z. Electrophoretic deposition of novel semi-permeable coatings on 3D-printed Ti-Nb alloy meshes for guided alveolar bone regeneration. Dent. Mater. 2022, 38, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, L.; Yan, D.; Shen, L. Surface Functionalization with Proanthocyanidins Provides an Anti-Oxidant Defense Mechanism That Improves the Long-Term Stability and Osteogenesis of Titanium Implants. Int. J. Nanomed. 2020, 15, 1643–1659. [Google Scholar] [CrossRef]
- Vakili, N.; Asefnejad, A. Titanium coating: Introducing an antibacterial and bioactive chitosan-alginate film on titanium by spin coating. Biomed. Eng. Biomed. Tech. 2020, 65, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Jabłoński, P.; Kyzioł, A.; Pawcenis, D.; Kyzioł, K. Electrostatic self-assembly approach in the deposition of bio-functional chitosan-based layers enriched with caffeic acid on Ti-6Al-7Nb alloys by alternate immersion. Biomater. Adv. 2022, 136, 212791. [Google Scholar] [CrossRef] [PubMed]
- Rahnamaee, S.Y.; Dehnavi, S.M.; Bagheri, R.; Karimi, A. Boosting bone cell growth using nanofibrous carboxymethylated cellulose and chitosan on titanium dioxide nanotube array with dual surface charges as a novel multifunctional bioimplant surface. Int. J. Biol. Macromol. 2023, 228, 570–581. [Google Scholar] [CrossRef]
- Ren, Y.; Qin, X.; Barbeck, M.; Liu, C. Mussel-Inspired Carboxymethyl Chitosan Hydrogel Coating of Titanium Alloy with Antibacterial and Bioactive Properties. Materials 2021, 14, 6901. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Vrana, N.E.; Dupret-Bories, A.; Bach, C.; Lavalle, P. Modification of macroporous titanium tracheal implants with biodegradable structures: Tracking in vivo integration for determination of optimal in situ epithelialization conditions. Biotechnol. Bioeng. 2012, 109, 2134–2146. [Google Scholar] [CrossRef]
- Gregurec, D.; Wang, G.; Pires, R.H.; Moya, S.E. Bioinspired titanium coatings: Self-assembly of collagen-alginate films for enhanced osseointegration. J. Mater. Chem. 2016, 4, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Shaygani, H.; Seifi, S.; Shamloo, A.; Ebrahimi, S. Novel bilayer coating on gentamicin-loaded titanium nanotube for orthopedic implants applications. Int. J. Pharm. 2023, 636, 122764. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wu, Y.; Yan, R.; Ma, H. Chitosan-sodium alginate-based coatings for self-strengthening anticorrosion and antibacterial protection of titanium substrate in artificial saliva. Int. J. Biol. Macromol. 2021, 184, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liu, P.; Hao, Y.; Cai, K. Construction of Ag-incorporated coating on Ti substrates for inhibited bacterial growth and enhanced osteoblast response. Colloid Surf. 2018, 171, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Zheng, S.; Shi, Z.; Cheng, H. Using a degradable three-layer sandwich-type coating to prevent titanium implant infection with the combined efficient bactericidal ability and fast immune remodeling property. Acta Biomater. 2022, 154, 650–666. [Google Scholar] [CrossRef]
- Perni, S.; Alotaibi, H.F.; Yergeshov, A.A.; Prokopovich, P. Long acting anti-infection constructs on titanium. J. Control. Release 2020, 326, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, S.; He, F.; Zou, S. Promotion of Osseointegration Using Protamine/Alginate/Bone Morphogenic Protein 2 Biofunctionalized Composite Coating on Nanopolymorphic Titanium Surfaces. J. Biomed. Nanotechnol. 2018, 14, 933–945. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Zhang, Y.; Lin, Y. Controlled release of dopamine coatings on titanium bidirectionally regulate osteoclastic and osteogenic response behaviors. Mat. Sci. Eng. C Mater. 2021, 129, 112376. [Google Scholar] [CrossRef]
- Chen, T.; Brial, C.; McCarthy, M.; Maher, S.A. Synthetic PVA Osteochondral Implants for the Knee Joint: Mechanical Characteristics during Simulated Gait. Am. J. Sport. Med. 2021, 49, 2933–2941. [Google Scholar] [CrossRef]
- Chen, K.; Liu, S.Y.; Wu, X.F.; Zhang, D.K. Mussel-inspired construction of Ti6Al4V-hydrogel artificial cartilage material with high strength and low friction. Mater. Lett. 2020, 265, 127421. [Google Scholar] [CrossRef]
- Cui, L.L.; Chen, J.Y.; Yan, C.Q.; Xiong, D.S. Articular Cartilage Inspired the Construction of LTi-DA-PVA Composite Structure with Excellent Surface Wettability and Low Friction Performance. Tribol. Lett. 2021, 69, 41. [Google Scholar] [CrossRef]
- Awasthi, S.; Gaur, J.K.; Pandey, S.K.; Srivastava, C. High-Strength, Strongly Bonded Nanocomposite Hydrogels for Cartilage Repair. ACS Appl. Mater. Interfaces 2021, 13, 24505–24523. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, J.; Li, J.L.; Xiong, D.S.; Dini, D. Improved mechanical and tribological properties of PAAm/PVA hydrogel-Ti6Al4V alloy configuration for cartilage repair. J. Polym. Res. 2022, 29, 515. [Google Scholar] [CrossRef]
- Deng, Y.L.; Sun, J.J.; Ni, X.Y.; Yu, B. Tribological properties of hierarchical structure artificial joints with poly acrylic acid (AA)-poly acrylamide (AAm) hydrogel and Ti6Al4V substrate. J. Polym. Res. 2020, 27, 157. [Google Scholar] [CrossRef]
- Chen, Y.H.; Cheng, W.H.; Teng, L.J.; Wang, Y.J. Graphene Oxide Hybrid Supramolecular Hydrogels with Self-Healable, Bioadhesive and Stimuli-Responsive Properties and Drug Delivery Application. Macromol. Mater. Eng. 2018, 303, 1700660. [Google Scholar] [CrossRef]
- Xupeng, G.; Fangyuan, Y.; Ming, C. Drug release kinetics of rifampicin from composite gel coating on surface of titanium alloy. Orthop. J. China 2018, 26, 649–654. [Google Scholar]
- Jing, Z.; Ni, R.; Wang, J.; Liu, Z. Practical strategy to construct anti-osteosarcoma bone substitutes by loading cisplatin into 3D-printed titanium alloy implants using a thermosensitive hydrogel. Bioact. Mater. 2021, 6, 4542–4557. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Fang, X.; Gao, X.; Qin, Y.G. Strong adhesive and drug-loaded hydrogels for enhancing bone-implant interface fixation and anti-infection properties. Colloid Surf. 2022, 219, 112817. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Yu, Z.; Yu, Q.; Yang, G. Electrochemical deposition of lithium coating on titanium implant with enhanced early stage osseointegration. J. Biomed. Mater. Res. 2022, 110, 2399–2410. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Chen, H.; Yuan, B.; Zhang, X. Electrochemical Deposition of Nanostructured Hydroxyapatite Coating on Titanium with Enhanced Early Stage Osteogenic Activity and Osseointegration. Int. J. Nanomed. 2020, 15, 6605–6618. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hua, J.; You, R.; Ma, L. Electrochemically deposition of catechol-chitosan hydrogel coating on coronary stent with robust copper ions immobilization capability and improved interfacial biological activity. Int. J. Biol. Macromol. 2021, 181, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Croes, M.; Bakhshandeh, S.; van Hengel, I.A.J.; Amin Yavari, S. Antibacterial and immunogenic behavior of silver coatings on additively manufactured porous titanium. Acta Biomater. 2018, 81, 315–327. [Google Scholar] [CrossRef]
- Yin, X.; Yan, L.; Jun Hao, D.; Liu, Z. Calcium alginate template-mineral substituted hydroxyapatite hydrogel coated titanium implant for tibia bone regeneration. Int. J. Pharm. 2020, 582, 119303. [Google Scholar] [CrossRef]
- Soylu, H.M.; Chevallier, P.; Copes, F.; Mantovani, D. A Novel Strategy to Coat Dopamine-Functionalized Titanium Surfaces with Agarose-Based Hydrogels for the Controlled Release of Gentamicin. Front. Cell. Infect. Microbiol. 2021, 11, 678081. [Google Scholar] [CrossRef]
- Yao, X.; Liu, J.; Yang, C.; Suo, Z. Hydrogel Paint. Adv. Mater. 2019, 31, e1903062. [Google Scholar] [CrossRef] [PubMed]
- Tolle, C.; Riedel, J.; Mikolai, C.; Menzel, H. Biocompatible Coatings from Smart Biopolymer Nanoparticles for Enzymatically Induced Drug Release. Biomolecules 2018, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Tsikopoulos, K.; Bidossi, A.; Drago, L.; Papaioannidou, P. Is Implant Coating with Tyrosol- and Antibiotic-loaded Hydrogel Effective in Reducing Cutibacterium (Propionibacterium) acnes Biofilm Formation? A Preliminary in Vitro Study. Clin. Orthop. Relat. Res. 2019, 477, 1736–1746. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, F.; Yang, X.; Zhang, X. Titanium surface polyethylene glycol hydrogel and gentamicin-loaded cross-linked starch microspheres release system for anti-infective drugs. J. Drug Target. 2023, 31, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; He, Y.; Yuan, Z.; Cai, K. Enzymatically-degradable hydrogel coatings on titanium for bacterial infection inhibition and enhanced soft tissue compatibility via a self-adaptive strategy. Bioact. Mater. 2021, 6, 4670–4685. [Google Scholar] [CrossRef]
- He, Y.; Li, K.; Yang, X.; Cai, K. Calcium Peroxide Nanoparticles-Embedded Coatings on Anti-Inflammatory TiO2 Nanotubes for Bacteria Elimination and Inflammatory Environment Amelioration. Small 2021, 17, e2102907. [Google Scholar] [CrossRef] [PubMed]
- Stoetzel, S.; Malhan, D.; Wild, U.; El Khassawna, T. Osteocytes Influence on Bone Matrix Integrity Affects Biomechanical Competence at Bone-Implant Interface of Bioactive-Coated Titanium Implants in Rat Tibiae. Int. J. Mol. Sci. 2021, 23, 374. [Google Scholar] [CrossRef]
- Shi, Q.; Qian, Z.; Liu, D.; Liu, H. Surface Modification of Dental Titanium Implant by Layer-by-Layer Electrostatic Self-Assembly. Front. Physiol. 2017, 8, 574. [Google Scholar] [CrossRef]
- Wang, D.; Chen, M.W.; Wei, Y.J.; Cai, K.Y. Construction of Wogonin Nanoparticle-Containing Strontium-Doped Nanoporous Structure on Titanium Surface to Promote Osteoporosis Fracture Repair. Adv. Healthc. Mater. 2022, 11, e2201405. [Google Scholar] [CrossRef]
- Lv, H.; Chen, Z.; Yang, X.; Gao, P. Layer-by-layer self-assembly of minocycline-loaded chitosan/alginate multilayer on titanium substrates to inhibit biofilm formation. J. Dent. 2014, 42, 1464–1472. [Google Scholar] [CrossRef]
- Chen, M.; Huang, L.; Shen, X.; Hu, Y. Construction of multilayered molecular reservoirs on a titanium alloy implant for combinational drug delivery to promote osseointegration in osteoporotic conditions. Acta Biomater. 2020, 105, 304–318. [Google Scholar] [CrossRef]
- Zhong, X.; Song, Y.; Yang, P.; Li, C. Titanium Surface Priming with Phase-Transited Lysozyme to Establish a Silver Nanoparticle-Loaded Chitosan/Hyaluronic Acid Antibacterial Multilayer via Layer-by-Layer Self-Assembly. PLoS ONE 2016, 11, e0146957. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ma, A.; Ning, J.; Li, C. Loading icariin on titanium surfaces by phase-transited lysozyme priming and layer-by-layer self-assembly of hyaluronic acid/chitosan to improve surface osteogenesis ability. Int. J. Nanomed. 2018, 13, 6751–6767. [Google Scholar] [CrossRef]
- Han, M.; Dong, Z.; Li, J.; Li, J. Mussel-inspired self-assembly engineered implant coatings for synergistic anti-infection and osteogenesis acceleration. J. Mater. Chem. 2021, 9, 8501–8511. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.; Nozaki, K.; Horiuchi, N.; Nagai, A. Effects of controlled micro-/nanosurfaces on osteoblast proliferation. J. Biomed. Mater. Res. 2017, 105, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jin, G.; Xue, Y.; Sun, J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017, 49, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chang, Y.Y.; Lee, J.; Shin, H. Surface engineering of titanium alloy using metal-polyphenol network coating with magnesium ions for improved osseointegration. Biomater. Sci. 2020, 8, 3404–3417. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tang, H.; Liu, L.; Wang, A. Biomimetic titanium implant coated with extracellular matrix enhances and accelerates osteogenesis. Nanomedicine 2020, 15, 1779–1793. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xiong, S.; Chen, Y.; Yang, B. Effects of statherin on the biological properties of titanium metals subjected to different surface modification. Colloid Surf. 2020, 188, 110783. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Lorusso, F.; Orsini, T.; Valbonetti, L. Biomimetic Surfaces Coated with Covalently Immobilized Collagen Type I: An X-ray Photoelectron Spectroscopy, Atomic Force Microscopy, Micro-CT and Histomorphometrical Study in Rabbits. Int. J. Mol. Sci. 2019, 20, 724. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, Y.; Yang, W.; Cai, K. Functionalization of titanium substrate with multifunctional peptide OGP-NAC for the regulation of osteoimmunology. Biomater. Sci. 2019, 7, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, X.; Yang, Y.; Zhang, X. Dual light-induced in situ antibacterial activities of biocompatibleTiO2/MoS2/PDA/RGD nanorod arrays on titanium. Biomater. Sci. 2020, 8, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.Y.; Tai, I.C.; Ho, M.L.; Tseng, C.C. Controlled release of BMP-2 from titanium with electrodeposition modification enhancing critical size bone formation. Mat. Sci. Eng. C Mater. 2019, 105, 109879. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, Y.; Zhang, W.; Shi, Q. Mussel-Inspired Peptide Coatings on Titanium Implant to Improve Osseointegration in Osteoporotic Condition. ACS Biomater. Sci. Eng. 2018, 4, 2505–2515. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Zhou, Y.; Zhang, Y. Biomimetic Ti-6Al-4V alloy/gelatin methacrylate hybrid scaffold with enhanced osteogenic and angiogenic capabilities for large bone defect restoration. Bioact. Mater. 2021, 6, 3437–3448. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, C.G.; Song, C.L. 3D printed porous titanium cages filled with simvastatin hydrogel promotes bone ingrowth and spinal fusion in rhesus macaques. Biomater. Sci. 2020, 8, 4147–4156. [Google Scholar] [CrossRef]
- He, P.; Zhang, H.; Li, Y.; Yang, S. 1α,25-Dihydroxyvitamin D3-loaded hierarchical titanium scaffold enhanced early osseointegration. Mat. Sci. Eng. C Mater. 2020, 109, 110551. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Cohen, D.J.; Sabalewski, E.L.; Boyan, B.D. Semaphorin 3A delivered by a rapidly polymerizing click hydrogel overcomes impaired implant osseointegration in a rat type 2 diabetes model. Acta Biomater. 2023, 157, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Mitchell, J.; Fernandez-Medina, T.; Ivanovski, S. Resorbable additively manufactured scaffold imparts dimensional stability to extraskeletally regenerated bone. Biomaterials 2021, 269, 120671. [Google Scholar] [CrossRef]
- Lovati, A.B.; Lopa, S.; Talò, G.; Moretti, M. In vivo evaluation of bone deposition in macroporous titanium implants loaded with mesenchymal stem cells and strontium-enriched hydrogel. J. Biomed. Mater. Res. 2015, 103, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Hossain, M.; Park, S.S.; Im, S.B.; Lee, B.T. Microstructures and biological properties of 3D-printed titanium intervertebral spacer with the tri-calcium phosphate loaded demineralized bone matrix hydrogel. Mater. Lett. 2021, 303, 130519. [Google Scholar] [CrossRef]
- Kwon, K.A.; Juhasz, J.A.; Brooks, R.A.; Best, S.M. Bioactive conformable hydrogel-carbonated hydroxyapatite nanocomposite coatings on Ti-6Al-4V substrates. Mater. Technol. 2020, 35, 727–733. [Google Scholar] [CrossRef]
- Alavi, S.E.; Panah, N.; Page, F.; Gholami, M. Hydrogel-based therapeutic coatings for dental implants. Eur. Polym. J. 2022, 181, 111652. [Google Scholar] [CrossRef]
- Lee, J.H.; Jin, Y.Z. Recombinant human bone morphogenetic protein-2 loaded porous beta-tricalcium phosphate microsphere-hyaluronic acid composites promoted osseointegration around titanium implants. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 368–374. [Google Scholar] [CrossRef]
- Kumar, A.; Nune, K.C.; Misra, R.D.K. Design and biological functionality of a novel hybrid Ti-6Al-4V/hydrogel system for reconstruction of bone defects. J. Tissue Eng. Regen. 2018, 12, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, W.; Liu, C.; Song, C.L. Incorporating simvastatin/poloxamer 407 hydrogel into 3D-printed porous Ti6Al4V scaffolds for the promotion of angiogenesis, osseointegration and bone ingrowth. Biofabrication 2016, 8, 045012. [Google Scholar] [CrossRef]
- Che, Z.J.; Sun, Y.F.; Luo, W.B.; Huang, L.F. Bifunctionalized hydrogels promote angiogenesis and osseointegration at the interface of three-dimensionally printed porous titanium scaffolds. Mater. Des. 2022, 223, 111118. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, S.; Zhao, L.; Zhuo, N. 3D printing of dual-cell delivery titanium alloy scaffolds for improving osseointegration through enhancing angiogenesis and osteogenesis. BMC Musculoskelet. Disord. 2021, 22, 734. [Google Scholar] [CrossRef]
- Li, B.E.; Zhang, L.; Wang, D.H.; Zhao, X.Y. Thermo-sensitive hydrogel on anodized titanium surface to regulate immune response. Surf. Coat. Technol. 2021, 405, 126624. [Google Scholar] [CrossRef]
- Gao, L.; Li, M.; Yin, L.; Feng, B. Dual-inflammatory cytokines on TiO2 nanotube-coated surfaces used for regulating macrophage polarization in bone implants. J. Biomed. Mater. Res. 2018, 106, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wei, F.; Yin, X.; Zhou, Y. Synergistic regulation of osteoimmune microenvironment by IL-4 and RGD to accelerate osteogenesis. Mat. Sci. Eng. C Mater. 2020, 109, 110508. [Google Scholar] [CrossRef]
- Chen, F.; He, Y.; Li, Z.; Zhang, Y. A novel tunable, highly biocompatible and injectable DNA-chitosan hybrid hydrogel fabricated by electrostatic interaction between chitosan and DNA backbone. Int. J. Pharm. 2021, 606, 120938. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, K.; He, Y.; Cai, K. ROS-responsive hydrogel coating modified titanium promotes vascularization and osteointegration of bone defects by orchestrating immunomodulation. Biomaterials 2022, 287, 121683. [Google Scholar] [CrossRef]
- Sun, C.K.; Ke, C.J.; Lin, Y.W.; Sun, J.S. Transglutaminase Cross-Linked Gelatin-Alginate-Antibacterial Hydrogel as the Drug Delivery-Coatings for Implant-Related Infections. Polymers 2021, 13, 414. [Google Scholar] [CrossRef]
- Huang, H.; Wu, Z.; Yang, Z.; Xie, X. In vitro application of drug-loaded hydrogel combined with 3D-printed porous scaffolds. Biomed. Mater. 2022, 17, 065019. [Google Scholar] [CrossRef] [PubMed]
- Boot, W.; Vogely, H.C.; Jiao, C.; Gawlitta, D. Prophylaxis of implant-related infections by local release of vancomycin from a hydrogel in rabbits. Eur. Cells Mater. 2020, 39, 108–120. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Chen, J.; Lai, M. Peptide GL13K releasing hydrogel functionalized micro/nanostructured titanium enhances its osteogenic and antibacterial activity. J. Biomat. Sci. Polym. 2022, 18, 1–17. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chiou, W.S.; Shiue, S.J.; Cheng, J.K. Non-RGD peptide H-ckrwwkwirw-NH2 grafting accentuates antibacterial and osteoinductive properties of biopolymer coating. Soft Mater. 2020, 18, 487–498. [Google Scholar] [CrossRef]
- Barros, J.A.R.; Melo, L.D.R.; Silva, R.A.R.D.; Monteiro, F.J. Encapsulated bacteriophages in alginate-nanohydroxyapatite hydrogel as a novel delivery system to prevent orthopedic implant-associated infections. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102145. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Wu, D.; Li, Z.; Gu, Y. The combination of multi-functional ingredients-loaded hydrogels and three-dimensional printed porous titanium alloys for infective bone defect treatment. J. Tissue Eng. 2020, 11, 2041731420965797. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, Y.; Wang, Z.; Wang, J. Engineering Multifunctional Hydrogel-Integrated 3D Printed Bioactive Prosthetic Interfaces for Osteoporotic Osseointegration. Adv. Healthc. Mater. 2022, 11, e2102535. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Li, B.; Wu, S. Near-Infrared Light Triggered Phototherapy and Immunotherapy for Elimination of Methicillin-Resistant Staphylococcus aureus Biofilm Infection on Bone Implant. ACS Nano 2020, 14, 8157–8170. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qiu, X.; Xia, T.; Li, Y. Mesoporous Materials Make Hydrogels More Powerful in Biomedicine. Gels 2023, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Andrade Del Olmo, J.; Pérez-Álvarez, L.; Sáez Martínez, V.; Alonso, J.M. Multifunctional antibacterial chitosan-based hydrogel coatings on Ti6Al4V biomaterial for biomedical implant applications. Int. J. Biol. Macromol. 2023, 231, 123328. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Chakravorty, N. Targeted Drug Delivery from Titanium Implants: A Review of Challenges and Approaches. Adv. Exp. Med. Biol. 2020, 1251, 1–17. [Google Scholar]
- Ding, Y.; Liu, G.; Liu, S.; Cai, K. A multi-function hydrogel-coating engineered implant for rescuing biofilm infection and boosting osseointegration by macrophage-related immunomodulation. Adv. Healthc. Mater. 2023, 4, e2300722. [Google Scholar] [CrossRef]










| Classification | Time | Representative Material | Advantage | Disadvantage |
|---|---|---|---|---|
| α | 1960s | Ti | Good biocompatibility | Low strength, poor wear resistance |
| α + β | 1970s | Ti6Al4V | Higher hardness, better wear resistance, lower elastic modulus, better mechanical compatibility | Biological toxicity of metal ions Al and V |
| 1980s | Ti6Al7Nb Ti5Al2.5Fe | Better biocompatibility | Easy corrosion, biological toxicity of Al metal ions | |
| β | 1990s | Ti13Nb13Zr Ti12Mo6Zr2Fe Ti15Mo | The low modulus of elasticity is close to that of human bones, non-biological toxicity of metal ions | Biological activity, abrasion resistance, and corrosion resistance still need to be improved |
| Classification | Representative Material | Advantage | Reference |
|---|---|---|---|
| Natural hydrogel coatings | Collagen-based | Improve the attachment of the peri-implant soft tissue to titanium at early stages | [15] |
| Enhance tissue vascularization and reduce inflammatory response | [16] | ||
| Improve gingival connective tissue response to titanium implants | [17] | ||
| Gelatin-based | Improve surface bio-activity | [18] | |
| Load with antibacterial agent curcumin | [19] | ||
| Chitosan-based | Enhance the antibacterial activity and osteoinductive properties | [20] | |
| Develop a close bony apposition or the osseointegration of dental/craniofacial and orthopedic implants | [21] | ||
| Provide a self-protective surface that prevents bacterial colonisation and implant-associated infections | [22] | ||
| Great potential in implant anticorrosion | [23] | ||
| Alginate-based | Improve the antibacterial effect and induce mineralization of dental implants | [24] | |
| Successively functionalize titanium surface | [25] | ||
| Synthetic hydrogels coatings | Polyvinyl alcohol | Improve the calcium silicate coating-to-substrate adhesion. | [26] |
| Polyacrylamide | Antimicrobial-loaded hydrogel coatings | [27] | |
| Polyethylene glycol | Lower albumin adsorption and presented a decreased fibroblast, Streptococcus sanguinis and Lactobacillus salivarius adhesion. | [28] | |
| Poly (lacto-glycolic acid) | Drug release | [29] | |
| Polyacrylic acid | Acts as both an effective bioactive surface and an effective anti-corrosion barrier | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Feng, R.; Xia, T.; Wen, Z.; Li, Q.; Qiu, X.; Huang, B.; Li, Y. Progress in Surface Modification of Titanium Implants by Hydrogel Coatings. Gels 2023, 9, 423. https://doi.org/10.3390/gels9050423
Chen H, Feng R, Xia T, Wen Z, Li Q, Qiu X, Huang B, Li Y. Progress in Surface Modification of Titanium Implants by Hydrogel Coatings. Gels. 2023; 9(5):423. https://doi.org/10.3390/gels9050423
Chicago/Turabian StyleChen, Huangqin, Rui Feng, Tian Xia, Zhehan Wen, Qing Li, Xin Qiu, Bin Huang, and Yuesheng Li. 2023. "Progress in Surface Modification of Titanium Implants by Hydrogel Coatings" Gels 9, no. 5: 423. https://doi.org/10.3390/gels9050423