Characterization of Essential Oils Obtained from Abruzzo Autochthonous Plants: Antioxidant and Antimicrobial Activities Assessment for Food Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
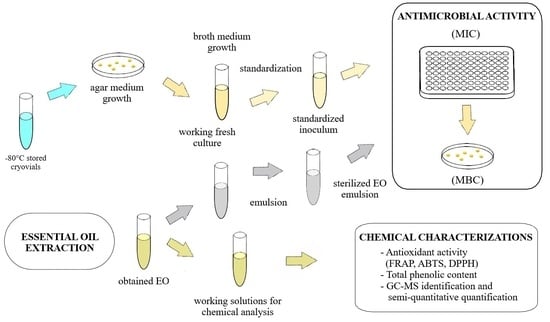
2.2. Essential Oils Extraction
2.3. Chemical Compositions of EOs
2.4. Antioxidant Capacity and Total Phenolic Content
2.4.1. Trolox Equivalent Antioxidant Capacity with 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid (TEAC/ABTS) Assay
2.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.4.3. 2,2-diphenyl-1-picrylhydrazyl (DPPH) Assay
2.4.4. Total Phenolic Content (TPC)
2.5. Antimicrobial Activity
2.5.1. Microbial Strains and Growth Conditions
2.5.2. Determination of Minimal Inhibitory Concentration
2.6. Statistical Analysis
3. Results & Discussion
3.1. Essential Oil Extractions
3.2. Chemical Characterization
3.3. Total Phenolic Content and Antioxidant Activity
3.4. Antimicrobial Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blackburn, C. Introduction. In Food Spoilage Microorganisms, 1st ed.; Blackburn, C., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2006; pp. xvii–xxiii. ISBN 9781845691417. [Google Scholar]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L.; Debabov, D. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157:H7, Salmonella Typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control 2007, 18, 414–420. [Google Scholar] [CrossRef]
- Tserennadmid, R.; Takó, M.; Galgóczy, L.; Papp, T.; Pesti, M.; Vágvölgyi, C.; Almássy, K.; Krisch, J. Anti yeast activities of some essential oils in growth medium, fruit juices and milk. Int. J. Food Microbiol. 2011, 144, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.L.; Lin, C.C.; Lin, W.C.; Yang, C.H. Antimicrobial, Antioxidant, and Anti-Inflammatory Activities of Essential Oils from Five Selected Herbs. Biosci. Biotechnol. Biochem. 2014, 75, 1977–1983. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. eCAM 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, J. Terpenoids as Plant Antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar] [PubMed]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Muthupandi, M.A.; Rajagopal, S.S. Phytochemical Evaluation and in vitro Antioxidant Activity of Various Solvent Extracts of Leucas aspera (Willd.) Link Leaves. Free Radic. Antioxid. 2017, 7, 166–171. [Google Scholar]
- Council of Europe. European Pharmacopoeia, 3rd ed.; Council of Europe: Strasbourg, France, 1997; ISBN 9287129916. [Google Scholar]
- Sefidkon, F. Influence of drying and extraction methods on yield and chemical composition of the essential oil of Satureja hortensis. Food Chem. 2006, 99, 19–23. [Google Scholar] [CrossRef]
- Şanli, A.; Karadoğan, T. Geographical Impact on Essential Oil Composition of Endemic Kundmannia anatolica Hub.-Mor. (Apiaceae). Afr. J. Tradit. Complement. Altern. Med. 2016, 14, 131–137. [Google Scholar]
- Silveri, A.; Manzi, A. Horticultural biodiversity and gardening in the region of Abruzzo. In Crop Genetic Resources in European Home Gardens, Proceedings of a Workshop, Ljubljana, Slovenia, 3–4 October 2007; Bailey, A., Eyzaguirre, P., Maggioni, L., Eds.; Bioversity International: Rome, Italy, 2009; pp. 26–36. [Google Scholar]
- Guarrera, P.M. Food medicine and minor nourishment in the folk traditions of Central Italy (Marche, Abruzzo ans Latium). Fitoterapia 2003, 74, 515–544. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz Navajas, Y.; Sánchez Zapata, E.; Fernández-lópez, J.; Pérez-álvarez, J.A. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Frag. J. 2009, 25, 13–19. [Google Scholar] [CrossRef]
- Hadjichambis, A.C.H.; Paraskeva-Hadjichambi, D.; Della, A.; Giusti, M.E.; De Pasquale, C.; Lenzarini, C.; Censorii, E.; Gonzales-Tejero, M.R.; Sanchez-Rojas, C.P.; Ramiro-Gutierrez, J.M.; et al. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int. J. Food Sci. Nutr. 2008, 59, 383–414. [Google Scholar] [CrossRef] [PubMed]
- Carrubba, A.; Scalenghe, R. The scent of Mare Nostrum: Medicinal and aromatic plants in Mediterranean soils. J. Sci. Food Agric. 2012, 92, 1150–1170. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.K.; Roy, J. Antimicrobial and Chemopreventive Properties of Herbs and Spices. Curr. Med. Chem. 2004, 11, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant Activity of Spices and Their Impact on Human Health: A Review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Sajid Arshad, M.; Ayesha Batool, S. Natural Antimicrobials, their Sources and Food Safety. In Food Additives; Karunaratne, D.N., Pamunuwa, G., Eds.; InTech: Rijeka, Croatia, 2017; pp. 87–102. [Google Scholar]
- Lee, L.M.; Vassilaros, D.L.; White, C.M. Retention indices for programmed-temperature capillary-column gas chromatography of polycyclic aromatic hydrocarbons. Anal. Chem. 1979, 51, 768–773. [Google Scholar] [CrossRef]
- Masaldan, S.; Iyer, V.V. Antioxidant and antiproliferative activities of methanolic extract of Aloe vera leaves in human cancer cell lines. J. Pharm. Res. 2011, 4, 2791–2796. [Google Scholar]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction prepared from glucosamine. Japan. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- El-Lateef Gharib, F.A.; Teixeira da Silva, J.A. Composition, Total Phenolic Content and Antioxidant Activity of the Essential Oil of Four Lamiaceae Herbs. Med. Aromat. Plant Sci. Biotechnol. 2013, 7, 19–27. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement; M100-S21; CLSI: Wayne, MI, USA, 2011. [Google Scholar]
- Rossi, C.; Chaves-López, C.; Serio, A.; Annibali, F.; Valbonetti, L.; Paparella, A. Effect of Origanum vulgare Essential Oil on Biofilm Formation and Motility Capacity of Pseudomonas fluorescens Strains Isolated from Discolored Mozzarella Cheese. J. Appl. Microbiol. 2018. Accepted. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, L.; Flamini, G.; Cioni, P.L.; Morelli, I. Composition and Antimicrobial Properties of Essential Oils of 4 Mediterranean Lamiaceae. J. Ethnopharmacol. 1993, 39, 167–170. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, J.G. Safety assessment of coriander (Coriandrum sativum L.) essential oil as a food ingredient. Food Chem. Toxicol. 2009, 47, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Galletti, G.C.; Bocchini, P.; Carnacini, A. Essential Oil Chemical Composition of Wild Populations of Italian Oregano Spice (Origanum vulgare ssp. hirtum (Link) Ietswaart): A Preliminary Evaluation of Their Use in Chemotaxonomy by Cluster Analysis. 1. Inflorescences. J. Food Chem. 1998, 8561, 3741–3746. [Google Scholar] [CrossRef]
- Sowbhagya, H.B.; Purnima, K.T.; Florence, S.P.; Rao, A.G.A.; Srinivas, P. Evaluation of enzyme-assisted extraction on quality of garlic volatile oil. Food Chem. 2009, 113, 1234–1238. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M. Characterization of several aromatic plants grown in northern Italy. Flavour Fragr. J. 1993, 8, 115–122. [Google Scholar] [CrossRef]
- Yesil Celiktas, O.; Hames Kocabas, E.E.; Bedir, E.; Vardar Sukan, F.; Ozek, T.; Baser, K.H.C. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem. 2007, 100, 553–559. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Cereti, E.; Barile, D.; Coïsson, J.D.; Arlorio, M.; Dessi, S.; Coroneo, V.; Cabras, P. Chemical Composition, Plant Genetic Differences, Antimicrobial and Antifungal Activity Investigation of the Essential Oil of Rosmarinus officinalis L. J. Agric. Food Chem. 2004, 52, 3530–3535. [Google Scholar] [CrossRef] [PubMed]
- Takayama, C.; Meira de-Faria, F.; Alves de Almeida, A.C.; Dunder, R.J.; Manzo, L.P.; Rabelo Socca, E.A.; Batista, L.M.; Salvador, M.J.; Monteiro Souza-Brito, A.R.; Luiz-Ferreira, A. Chemical composition of Rosmarinus officinalis essential oil and antioxidant action against gastric damage induced by absolute ethanol in the rat. Asian Pac. J. Trop. Biomed. 2016, 6, 677–681. [Google Scholar] [CrossRef]
- Pintore, G.; Usai, M.; Bradesi, P.; Juliano, C.; Boatto, G.; Tomi, F.; Chessa, M.; Cerri, R.; Casanova, J. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. oils from Sardinia and Corsica. Flavour Fragr. J. 2002, 17, 15–19. [Google Scholar] [CrossRef]
- Lo Presti, M.; Ragusa, S.; Trozzi, A.; Dugo, P.; Visinoni, F.; Fazio, A.; Dugo, G.; Mondello, L. A comparison between different techniques for the isolation of rosemary essential oil. J. Sep. Sci. 2005, 28, 273–280. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; De Feo, V.; Formisano, C.; Mignola, E.; Senatore, F. Chemical composition and antimicrobial activity of the essential oils from three chemotypes of Origanum vulgare L. ssp. hirtum (Link) Ietswaart growing wild in campania (Southern Italy). Molecules 2009, 14, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Vazirian, M.; Mohammadi, M.; Farzaei, M.H.; Amin, G.; Amanzadeh, Y. Chemical composition and antioxidant activity of Origanum vulgare subsp. vulgare essential oil from Iran. Res. J. Pharmacogn. 2015, 2, 41–46. [Google Scholar]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J. Agric. Food Chem. 2000, 48, 2576–2581. [Google Scholar] [CrossRef] [PubMed]
- Dadalioglu, I.; Evrendilek, G.A. Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8255–8260. [Google Scholar] [CrossRef] [PubMed]
- Mazzarrino, G.; Paparella, A.; Chaves-López, C.; Faberi, A.; Sergi, M.; Sigismondi, C.; Compagnone, D.; Serio, A. Salmonella enterica and Listeria monocytogenes inactivation dynamics after treatment with selected essential oils. Food Control 2015, 50, 794–803. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Arak, E. Composition of the essential oil of Salvia officinalis L. from various European countries. Nat. Prod. Res. 2007, 21, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Badiee, P.; Nasirzadeh, A.R.; Motaffaf, M. Comparison of Salvia officinalis L. essential oil and antifungal agents against candida species. J. Pharm. Technol. Drug Res. 2012, 1, 7. [Google Scholar] [CrossRef]
- Dziri, S.; Casabianca, H.; Hanchi, B.; Hosni, K. Composition of garlic essential oil (Allium sativum L.) as influenced by drying method. J. Essent. Oil Res. 2014, 26, 91–96. [Google Scholar] [CrossRef]
- Yu, T.H.; Wu, C.M.; Liou, Y.C. Volatile Compounds from Garlic. J. Agric. Food Chem. 1989, 37, 725–730. [Google Scholar] [CrossRef]
- Thompson, J.D.; Chalchat, J.C.; Michet, A.; Linhart, Y.B.; Ehlers, B. Qualitative and Quantitative Variation in Monoterpene co-Occurrence and Composition in the Essential Oil of Thymus vulgaris Chemotypes. J. Chem. Ecol. 2003, 29, 859–880. [Google Scholar] [CrossRef] [PubMed]
- Piccaglia, R.; Marotti, M. Characterization of some Italian types of wild fennel (Foeniculum vulgare Mill.). J. Agric. Food Chem. 2001, 49, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PLoS ONE 2014, 9, e114767. [Google Scholar] [CrossRef] [PubMed]
- Saharkhiz, M.J.; Motamedi, M.; Zomorodian, K.; Pakshir, K.; Miri, R.; Hemyari, K. Chemical Composition, Antifungal and Antibiofilm Activities of the Essential Oil of Mentha piperita L. ISRN Pharm. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mandal, M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pac. J. Trop. Biomed. 2015, 5, 421–428. [Google Scholar] [CrossRef]
- Miladi, H.; Slama, R.B.; Mili, D.; Zouari, S.; Bakhrouf, A.; Ammar, E. Chemical Composition and Cytotoxic and Antioxidant Activities of Satureja montana L. Essential Oil and Its Antibacterial Potential against Salmonella Spp. Strains. J. Chem. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Lo Cantore, P.; Iacobellis, N.S.; De Marco, A.; Capasso, F.; Senatore, F. Antibacterial activity of Coriandrum sativum L. and Foeniculum vulgare Miller Var. vulgare (Miller) essential oils. J. Agric. Food Chem. 2004, 52, 7862–7866. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Hway, K.H.; Yang, C.H.; Huang, K.F. Anti-oxidant activity and major chemical component analyses of twenty-six commercially available essential oils. J. Food Drug Anal. 2017, 25, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Çetin, B.; Özer, H.; Cakir, A.; Polat, T.; Dursun, A.; Mete, E.; Öztürk, E.; Ekinci, M. Antimicrobial Activities of Essential Oil and Hexane Extract of Florence Fennel [Foeniculum vulgare var. azoricum (Mill.) Thell.] Against Foodborne Microorganisms. J. Med. Food 2010, 13, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M.; Kazempour, N.; Mahboubi, M. Antimicrobial activity of Rosemary, Fennel and Galbanum essential oils against clinical isolates of Staphylococcus aureus. Biharean Biol. 2011, 5, 4–7. [Google Scholar]
- Tarek, N.; Hassan, H.M.; AbdelGhani, S.M.M.; Radwan, I.A.; Hammouda, O.; El-Gendy, A.O. Comparative chemical and antimicrobial study of nine essential oils obtained from medicinal plants growing in Egypt. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 149–156. [Google Scholar] [CrossRef]
- Gachkar, L.; Yadegari, D.; Bagher, M.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Food Chemistry Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chem. 2007, 102, 898–904. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Pirbalouti, A.G.; Rahimmalek, M.; Malekpoor, F.; Karimi, A. Variation in antibacterial activity, thymol and carvacrol contents of wild populations of Thymus daenensis subsp. daenensis Celak. Plant Omics 2011, 4, 209–214. [Google Scholar]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fraction of dill, celandra, coriander and eucalyptus essential oil. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- Paparella, A.; Taccogna, L.; Aguzzi, I.; Chaves-López, C.; Serio, A.; Marsilio, F.; Suzzi, G. Flow cytometric assessment of the antimicrobial activity of essential oils against Listeria monocytogenes. Food Control 2008, 19, 1174–1182. [Google Scholar] [CrossRef]
- Serio, A.; Chiarini, M.; Tettamanti, E.; Paparella, A. Electronic paramagnetic resonance investigation of the activity of Origanum vulgare L. essential oil on the Listeria monocytogenes membrane. Lett. Appl. Microbiol. 2010, 51, 149–157. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, S.; Serio, A.; Chaves-López, C.; Paparella, A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control 2018, 86, 126–137. [Google Scholar] [CrossRef]
| Strains | Origin | Incubation Temperature (°C) | Incubation Time (h) | |
|---|---|---|---|---|
| Pseudomonas fluorescens | P34 | Dairy products | 28 | 24 |
| Brochothrix thermosphacta | B2 | Poultry meat | 30 | 48 |
| Brochothrix thermosphacta | B1 | Poultry meat | 30 | 48 |
| Salmonella Enteritidis | S2 | Meat | 37 | 24 |
| Salmonella Typhimurium | S4 | Meat | 37 | 24 |
| Enterococcus faecium | P14 | Fish | 30 | 48 |
| Enterococcus faecium | ATCC 19434 | Type strain | 30 | 48 |
| Listeria monocytogenes | LM 4 | Meat products | 37 | 48 |
| Listeria monocytogenes | ATCC 19144 | Type strain | 37 | 48 |
| Listeria monocytogenes | ATCC 7644 | Type strain | 37 | 48 |
| Staphylococcus aureus | STA 32 | Dairy products | 37 | 48 |
| Staphylococcus aureus | STA 47 | Dairy products | 37 | 48 |
| Staphylococcus aureus | STA 39 | Dairy products | 37 | 48 |
| ID | RID | RIE | R. officinalis | O. vulgare | S. officinalis | A. sativum | F. vulgare | M. piperita | C. sativum | S. montana | T. vulgaris |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diallyl sulfide | 848 | 847 | - | - | - | 0.55 ± 0.04 c | - | - | - | - | - |
| Methyl allyl disulfide | 910 | 911 | - | - | - | 0.29 ± 0.00 c | - | - | - | - | - |
| α-Pinene | 939 | 939 | 16.64 ± 0.22 b | 0.47 ± 0.02 i | 1.20 ± 0.10 f,g | - | 5.18 ± 0.73 c | 0.86 ± 0.03 e | 0.28 ± 0.00 e | - | 0.37 ± 0.01 f,g |
| Camphene | 953 | 956 | 3.39 ± 0.04 f | - | 1.38 ± 0.03 f | - | - | - | - | - | - |
| Thuja-2,4(10)-diene | 957 | 960 | 0.32 ± 0.01 l | - | - | - | - | - | - | - | - |
| 1-Octen-3-ol | 978 | 979 | - | 0.57 ± 0.02 h,i | - | - | - | - | - | 1.43 ± 0.02 e | - |
| β-Pinene | 979 | 981 | 2.35 ± 0.03 g | - | 2.77 ± 0.16 e | - | 1.03 ± 0.05 e | 0.52 ± 0.01 e | - | - | 1.87 ± 0.09 f |
| β-Myrcene | 992 | 993 | 0.72 ± 0.01 k | - | - | - | 0.65 ± 0.06 e | - | 0.28 ± 0.03 e | 0.55 ± 0.03 e | 0.56 ± 0.02 g,h |
| α-Phellandrene | 1005 | 1006 | - | - | - | - | 10.49 ± 0.02 b | - | - | - | - |
| trans-β-Ocimene | 1015 | 1014 | 0.38 ± 0.02 l | - | - | - | - | - | - | - | - |
| p-Cymene | 1024 | 1021 | 1.75 ± 0.01 i | 8.30 ± 0.02 c | 0.52 ± 0.03 g | - | 3.33 ± 0.24 d | - | 0.31 ± 0.00 e | 10.78 ± 0.41 b | 18.57 ± 0.71 b |
| Limonene | 1029 | 1029 | - | - | - | 4.56 ± 0.38 c,d | - | 0.33 ± 0.00 e | 0.69 ± 0.04 e | - | |
| 1,8-Cineole | 1032 | 1034 | 15.71 ± 0.18 c | - | 10.02 ± 0.36 c | - | - | 5.35 ± 0.38 c,d | - | 0.53 ± 0.01 e | - |
| γ-Terpinene | 1060 | 1061 | 0.46 ± 0.02 k,l | 9.38 ± 0.01 c | - | - | - | - | 1.19 ± 0.07 d,e | 6.46 ± 0.71 c | 4.92 ± 0.13 c,d |
| cis-Sabinene hydrate | 1066 | 1064 | - | - | - | - | - | - | - | - | 3.17 ± 0.03 e |
| Diallyl disulfide | 1080 | 1079 | - | - | - | 20.16 ± 2.84 b | - | - | - | - | - |
| Terpinolene | 1087 | 1085 | - | - | - | - | - | - | - | - | 4.54 ± 0.06 d |
| Fenchone | 1088 | 1088 | - | - | - | - | 10.12 ± 0.07 b | - | - | - | - |
| (S)-(+)-Linalool | 1100 | 1099 | 2.02 ± 0.01 h | 1.95 ± 0.10 e,f | - | - | - | 0.86 ± 0.04 e | 77.07 ± 1.82 a | 2.09 ± 0.10 d,e | 0.37 ± 0.01 g,h |
| α-Thujone | 1102 | 1104 | 30.46 ± 0.49 a | ||||||||
| 1-Octen-3-ol, acetate | 1110 | 1113 | - | - | - | - | - | - | - | - | 1.27 ± 0.01 f,g |
| Menthone | 1126 | 1116 | - | - | - | - | - | 6.87 ± 0.11 b,c | - | - | - |
| l-Pinocarveol | 1135 | 1132 | 0.23 ± 0.01 l | - | - | - | - | - | - | - | - |
| Camphor | 1139 | 1137 | 22.07 ± 0.23 a | - | 11.53 ± 0.34 b | - | - | - | 2.60 ± 0.10 c,d | - | 0.29 ± 0.02 h |
| Borneol | 1162 | 1160 | 11.99 ± 0.04 d | 0.66 ± 0.01 g,h,i | 3.92 ± 0.02 d | - | - | - | 0.48 ± 0.01 e | 4.51 ± 0.37 c,d | - |
| Menthofuran | 1164 | 1165 | - | - | - | - | - | 7.81 ± 0.59 b | - | - | - |
| Menthol | 1171 | 1178 | - | - | - | - | - | 53.39 ± 0.24 a | - | - | - |
| Isocamphopinone | 1175 | 1174 | 1.08 ± 0.00 j | - | - | - | - | - | - | - | - |
| Terpinene-4-ol | 1179 | 1176 | 0.26 ± 0.01 l | 0.38 ± 0.01 i | 1.43 ± 0.09 f | - | - | - | 0.55 ± 0.03 e | - | - |
| 2-Vinyl-1.3-dithiane | 1182 | 1084 | - | - | - | 4.60 ± 0.09 c | - | - | - | - | - |
| Isomenthol | 1194 | 1192 | - | - | - | - | - | - | - | - | - |
| α-Terpineol | 1195 | 1195 | 1.49 ± 0.08 i | 1.64 ± 0.04 f,g,h,i | 0.51 ± 0.01 g | - | 0.49 ± 0.03 e | - | 0.59 ± 0.03 e | 1.61 ± 0.02 e | 4.48 ± 0.09 d |
| Myrtenol | 1196 | 1194 | 2.35 ± 0.03 g | - | - | - | - | - | - | - | 2.82 ± 0.15 e |
| Estragole | 1199 | 1198 | - | - | - | - | 44.86 ± 0.26 a | - | - | - | |
| Verbenone | 1205 | 1203 | 0.44 ± 0.05 l | - | - | - | - | - | - | - | - |
| Isopulegone | 1237 | 1237 | - | - | - | - | - | 2.01 ± 0.03 e | - | - | - |
| Piperitone | 1253 | 1250 | - | - | - | - | - | 0.21 ± 0.02 e | - | - | - |
| cis-Geraniol | 1254 | 1256 | - | - | - | - | - | - | 5.24 ± 0.39 b | - | - |
| Thymol methyl ether | 1255 | 1257 | - | 1.04 ± 0.01 f,g,h | - | - | - | - | - | - | - |
| Neomenthyl acetate | 1273 | 1270 | - | - | - | - | - | 0.81 ± 0.06 e | - | - | - |
| trans-anethol | 1285 | 1282 | - | - | - | 6.55 ± 0.06 c | - | - | - | - | |
| Bornyl acetate | 1286 | 1286 | 5.62 ± 0.01 e | - | - | - | - | - | - | - | - |
| Thymol | 1290 | 1290 | - | 40.32 ± 1.12 a | - | - | - | - | - | 2.53 ± 0.14 d,e | 43.68 ± 0.54 a |
| Menthyl acetate | 1295 | 1294 | - | - | - | - | - | 4.61 ± 0.01 d | - | - | - |
| Carvacrol | 1299 | 1299 | - | 16.20 ± 0.05 b | - | - | 0.63 ± 0.01 e | - | 1.03 ± 0.03 d,e | 54.17 ± 2.33 a | 5.51 ± 0.12 c |
| Diallyl trisulfide | 1301 | 1300 | - | - | - | 65.39 ± 2.50 a | - | - | - | - | - |
| Isocaryophyllene | 1438 | 1434 | 3.37 ± 0.20 f | 2.90 ± 0.07 e | 10.49 ± 0.00 c | - | 1.05 ± 0.05 e | 1.67 ± 0.03 e | 0.62 ± 0.02 e | 2.91 ± 0.11 d,e | 1.61 ± 0.03 f |
| Humulene | 1467 | 1467 | 0.72 ± 0.02 k | 0.77 ± 0.03 g,h,i | 10.01 ± 0.35 c | - | 0.49 ± 0.01 e | - | - | - | - |
| Germacrene-d | 1487 | 1490 | - | 0.90 ± 0.01 f,g,h,i | - | - | - | 0.87 ± 0.02 e | - | - | 0.55 ± 0.01 g,h |
| β-Bisabolene | 1506 | 1509 | - | 4.64 ± 0.06 d | - | - | - | - | 0.83 ± 0.04 d,e | 1.82 ± 0.03 e | 1.64 ± 0.01 f |
| γ-Cadinene | 1513 | 1514 | - | - | - | - | - | - | - | 0.54 ± 0.02 e | - |
| δ-Cadinene | 1523 | 1522 | - | 0.65 ± 0.01 g,h,i | 0.45 ± 0.01 g | - | - | - | - | 0.54 ± 0.02 e | - |
| Diallyl tetrasulfide | 1555 | 1557 | - | - | - | 1.49 ± 0.07 c | - | - | - | - | - |
| Caryophyllene oxide | 1581 | 1583 | - | 1.73 ± 0.01 f,g | 1.92 ± 0.05 e,f | - | - | 1.21 ± 0.04 e | 3.16 ± 0.17 c | 1.41 ± 0.05 e | - |
| Ledene | 1585 | 1589 | - | - | 10.12 ± 0.44 c | - | - | - | - | - | - |
| Total identified compounds | 93.35 ± 0.43 | 92.52 ± 0.84 | 97.04 ± 0.46 | 92.47 ± 0.39 | 90.44 ± 0.87 | 93.17 ± 0.39 | 94.55 ± 1.69 | 92.56 ± 3.94 | 96.22 ± 1.47 | ||
| Assay | TPC | FRAP | DPPH | ABTS | ||
|---|---|---|---|---|---|---|
| Rosmarinus officinalis | 0.111 ± 0.002 c | 188.270 ± 0.437 a | 10.288 ± 0.258 c,d | 0.084 ± 0.001 e | ||
| Origanum vulgare | 4.688 ± 0.304 b | 168.220 ± 1.837 b | 23.963 ± 2.435 b | 1.765 ± 0.005 b | ||
| Salvia officinalis | 0.178 ± 0.008 c | 12.304 ± 0.022 f | 8.709 ± 0.885 c,d | 0.098 ± 0.005 e | ||
| Mentha piperita | 0.338 ± 0.018 c | 0.543 ± 0.044 h | 11.289 ± 0.514 c | 0.154 ± 0.006 d | ||
| Allium sativum | 0.050 ± 0.001 c | 3.924 ± 0.142 g | 7.868 ± 0.158 d | 0.037 ± 0.003 g | ||
| Foeniculum vulgare | 0.283 ± 0.013 c | 15.202 ± 0.175 e | 11.466 ± 0.636 c | 0.043 ± 0.003 g | ||
| Coriandrum sativum | 0.046 ± 0.004 c | 4.122 ± 0.241 g | 10.656 ± 1.043 c,d | 0.067 ± 0.004 f | ||
| Satureja montana | 4.398 ± 0.252 b | 159.280 ± 1.575 c | 27.015 ± 0.959 a | 1.997 ± 0.003 a | ||
| Thymus vulgaris | 6.419 ± 0.219 a | 126.869 ± 0.175 d | 21.751 ± 0.862 b | 1.131 ± 0.012 c | ||
| Pearson Correlation Coefficients | ||||||
| Assay | FRAP | ABTS | DPPH | |||
| TPC | 0.642 | 0.691 | 0.905 | |||
| Strains | Rosmarinus officinalis | Origanum vulgare | Salvia officinalis | Mentha piperita | Allium sativum | Foeniculum vulgare | Satureja montana | Thymus vulgaris | Coriandrum sativum | |
|---|---|---|---|---|---|---|---|---|---|---|
| P. fluorescens | P34 | 10 | 1.25 | 10 | >20 | 10 | >20 | 1.25 | 2.5 | 5 |
| B. thermosphacta | B2 | >20 | 10 | >20 | >20 | >20 | >20 | 2.5 | 2.5 | 5 |
| B. thermosphacta | B1 | >20 | 10 | >20 | >20 | >20 | >20 | 5 | 2.5 | 5 |
| S. Enteritidis | S2 | 20 | 2.5 | >20 | >20 | >20 | >20 | 1.25 | 1.25 | 5 |
| S. Typhimurium | S4 | >20 | 2.5 | >20 | >20 | >20 | >20 | 5 | 2.5 | 5 |
| E. faecium | P14 | >20 | 5 | >20 | >20 | >20 | >20 | 5 | 5 | 5 |
| E. faecium | ATCC 19434 | 10 | 5 | >20 | >20 | 10 | >20 | 5 | 2.5 | 2.5 |
| L. monocytogenes | LM 4 | >20 | 5 | 10 | >20 | >20 | >20 | 2.5 | 1.25 | 0.625 |
| L. monocytogenes | ATCC 19144 | 10 | 5 | 5 | 20 | 2.5 | >20 | 2.5 | 1.25 | 0.625 |
| L. monocytogenes | ATCC 7644 | 5 | 10 | 10 | 10 | 2.5 | >20 | 5 | 1.25 | 0.625 |
| S. aureus | STA 32 | 10 | 5 | >20 | >20 | 10 | >20 | 2.5 | 2.5 | 0.625 |
| S. aureus | STA 47 | 5 | 5 | 10 | 2.5 | 5 | >20 | 5 | 1.25 | 1.25 |
| S. aureus | STA 39 | >20 | 5 | >20 | >20 | 10 | >20 | 5 | 1.25 | 1.25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrini, M.; Ricci, A.; Serio, A.; Chaves-López, C.; Mazzarrino, G.; D’Amato, S.; Lo Sterzo, C.; Paparella, A. Characterization of Essential Oils Obtained from Abruzzo Autochthonous Plants: Antioxidant and Antimicrobial Activities Assessment for Food Application. Foods 2018, 7, 19. https://doi.org/10.3390/foods7020019
Pellegrini M, Ricci A, Serio A, Chaves-López C, Mazzarrino G, D’Amato S, Lo Sterzo C, Paparella A. Characterization of Essential Oils Obtained from Abruzzo Autochthonous Plants: Antioxidant and Antimicrobial Activities Assessment for Food Application. Foods. 2018; 7(2):19. https://doi.org/10.3390/foods7020019
Chicago/Turabian StylePellegrini, Marika, Antonella Ricci, Annalisa Serio, Clemencia Chaves-López, Giovanni Mazzarrino, Serena D’Amato, Claudio Lo Sterzo, and Antonello Paparella. 2018. "Characterization of Essential Oils Obtained from Abruzzo Autochthonous Plants: Antioxidant and Antimicrobial Activities Assessment for Food Application" Foods 7, no. 2: 19. https://doi.org/10.3390/foods7020019